Abstract
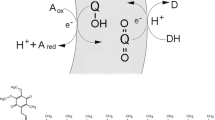
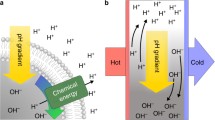
A critical phase in the transition from prebiotic chemistry to biological evolution was apparently an asymmetric ion flow across the lipid membrane. Due to imbalance in the ion flow, the early lipid vesicles could selectively take the necessary molecules from the environment, and release the side-products from the vesicle. Natural proton gradients played a definitively crucial role in this process, since they remain the basis of energy transfer in the present-day cells. On the basis of this supposition, and the premise of the early vesicle membrane’s impermeability to protons, we have shown that the emergence of the proton gradient in the lipid vesicle could be a key physical factor in the evolution of the forced transport mechanism (pore formation and active transport) across the lipid bilayer. This driven flow of protons across the membrane is the result of the electrochemical proton gradient and osmotic pressures on the integrity of the lipid vesicle. At a critical number of new lipid molecules incorporated into the vesicle, the energies associated with the creation of the proton gradient exceed the bending stiffness of the lipid membrane, and overlap the free energy of the lipid bilayer pore formation.
Similar content being viewed by others
Introduction
It is obvious that the first simple primitive forms of life on Earth (protocells) would have consisted of at least one key component: a lipid membrane that spatially defined the protocell in the environment. In order to understand the origins of a protocell it is necessary to identify the physico-chemical conditions that enabled early lipid vesicles to selectively capture the necessary molecules from the environment, and release side-products. Such an emergence of the asymmetric flow of ions and molecules across the lipid vesicle membranes appears to represent a critical step in the transition from prebiotic chemistry to biological evolution. Such asymmetry could arise from the selective permeability of the lipid bilayer to a different types of ions and molecules. In accordance with the recent study of Sojo et al. (2014), we suppose that a key component of this asymmetry is a proton flow imbalance through the lipid membrane. The electrochemical proton gradients remain the backbone of the energy transfer in present-day cells, with the transduction of the energy representing a fundamental step in Darwinian evolution.
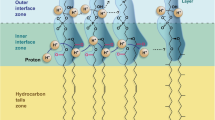
Current cell membranes are composed almost exclusively of diacyl or dialkyl glycerol phospholipids, and sterols as well as various kinds of proteins. However, early cell membranes were very likely composed of simple single-chain lipids that were present in the prebiotic environment. Short-chain fatty acids and their derivatives are ideal analogues of protocellular membranes (Hanczyz et al. 2003), primarily due to their autocatalytic self-assembly behaviour (Berclaz et al. 2001) and cyclical growth and division (Hanczyz et al. 2003). Moreover, fatty acids have been found in CM2 Murchison meteorite (Deamer 1985), one of the carbonaceous chondrites that represent the most chemically primitive material in the solar system. Fatty acids can also be synthesised under simulated prebiotic conditions (Rushdi and Simoneit 2001). The principal inconvenience of pure fatty acid vesicles is their inability to maintain a stable pH gradient, as they are highly permeable to protons. It was revealed that an addition of merely a small amount of oleic acid to phospholipid vesicles results in the dissipation of pre-established pH gradients within several seconds (Pohl et al. 2000). However, Chen and Szostak (2004) prepared fatty acid vesicles capable of maintaining a pH gradient, with the gradient established as a result of vesicles’ growth, where protonated fatty acid molecules crossed the membrane and released protons into their interior. Moreover, from an origin-of-life perspective, such energy storage in the formation of pH gradient during membrane growth is one of the most interesting properties of fatty acid vesicles, and the initial focus of our simulations. Our aim is to demonstrate that the emergence of the pH gradient in early lipid membranes—protocells (as a consequence of their growth)—could be a starting point in the evolution of the forced (pores’ formation and active transport) transport mechanism across the lipid bilayer.
Simulation and Calculation Methods
We simulated the pH decrease (Eq. 3) inside the lipid vesicle, dependent upon the number of incorporated lipid molecules from the environment. Energies associated with the pH decrease and consequent pH gradient and osmotic pressure emergence through the temperature range of 0–100 °C were determined and compared with the biological values of the forced transport mechanism across the lipid bilayer. Our simulations followed on from the work of Chen and Szostak (2004), in which the new lipid molecules (micelle) were incorporated into the bilayer membrane of the (fatty acid) lipid vesicle (LV) rLV = 100 nm in size. Studies on small (≈ 100 nm) fatty acid vesicles highlighted that the addition of fatty-acid micelles led to vesicle growth with an efficiency of ≈ 90 % (Hanczyz et al. 2003). The initial proton concentrations outside (in the proximity of the membrane surface) and inside the lipid bilayer of the former lipid vesicle are equal. The incorporation (fusion) of the new lipid molecules into the lipid vesicle membrane results in the pH decrease inside the vesicle as a consequence of the flip-flop mechanism of the incorporated lipids (Chen and Szostak 2004). The pH decrease inside the vesicle establishes an electrochemical proton gradient across the lipid bilayer that is impermeable for protons (see Premise 2). The pH gradient is associated with the creation of free Gibbs energy (ΔGgrad) that forces the protons to leave the vesicle. Moreover, the membrane’s impermeability leads to osmosis that results in additional pressure on the vesicle’s membrane (Chen et al. 2004). The water molecules can traverse the monoacyl as well as the diacyl lipid membrane with the same (high) permeability coefficient (Mansy 2010). Osmotic pressure together with the electrochemical gradient strain the vesicle membrane, with the overpressure (at a critical number of incorporated lipid molecules) having the potential to lead to the disruption of the lipid bilayer—the bending stiffness of typical biological membranes is in the order of 10−19 J (Picas et al. 2012). Internal pressure that is higher than the bending stiffness value of the lipid bilayer can result in the formation of pores in the membrane, and/or the evolution of an active transport mechanism of protons across the membrane, as a consequence of early lipid vesicles physico-chemical selection, where the integrity of the lipid vesicle in the aqueous medium is defaulted as being naturally selected (i.e. energetically most favourable; see Premise 3).
Premises
We have accepted the following premises:
-
(1)
Proton gradients are the basis of asymmetric ion flow across the early lipid membranes
The survival of protocells depended very probably upon the existence of natural proton gradients, as the ATP synthesis of every current cell type is powered by electrochemical differences in the concentration of H+ and/or Na+ ions across membranes (Mitchell 1961). Moreover, the protons permeate the lipid bilayer at a much higher rate than other small ions (Tepper and Voth 2005).
-
(2)
Early lipid membranes were impermeable to protons
The early lipid vesicles were almost certainly composed of mixtures of amphiphilic molecules. The membrane of such vesicles is less permeable to protons than a membrane composed of single pure spices. Proton impermeable vesicles could emerge as a consequence of the incorporation of stabilising compounds from the environment into the pure lipid vesicles. Such stabilising compounds include fatty acid alcohols and monoacyl glycerol derivatives (Maurer et al. 2009), mixed cationic and anionic amphiphiles (Namani and Deamer 2008), polycyclic aromatic hydrocarbons (Groen et al. 2012), and phospholipids. Budin and Szostak (2011) demonstrated that already low levels of phospholipids can drive protocell membrane growth during competition for single-chain lipids. They showed that the resulting increase in membrane phospholipids’ content would have led to a cascade of new selective pressures for the evolution of metabolic and transport machinery to overcome the reduced membrane permeability of the diacyl lipid membranes.
The presence of new amphiphiles could be a result of synthesis via Fischer–Tropsch reactions associated with volcanism (McCollom and Seewald 2007), or through the delivery of extraterrestrial organic compounds during a heavy bombardment phase of the young Earth (Chyba and Sagan 1992). Recent experiments have revealed that the physical state and/or composition of the vesicle membrane can drive competition for simple amphiphilic molecules (Adamala and Szostak 2013; Budin and Szostak 2011; Chen et al. 2004; Cheng and Luisi 2003). Thus, if lipid resources are limited, lipid vesicles steal lipids from one another. Such competition in the protocellular world could result in Darwinian natural selection capable of Darwinian evolution (Munteanu et al. 2007). It has been calculated that diacyl phospholipids are taken up from the solution by the lipid vesicle membrane at a rate five orders of magnitude slower than monoacyl fatty acids (Mavelli and Ruiz-Mirazo 2010). Moreover, they have higher flip-flop dynamics than phospholipids (Chen and Szostak 2004), while together with the selective permeability of fatty acid membranes to charged and less polar molecules (Mansy 2010), the incorporation of new lipid molecules results in a decrease of pH in the vesicles interior and the emergence of a pH gradient across the membrane.
-
(3)
The integrity of the lipid vesicle in an aqueous medium is energetically the most favourable state (naturally selected at the chemical level)
The uncontrollable increase of proton concentration inside the vesicles can result in vesicle disruption due to osmotic and electrochemical gradient pressures. To prevent the collapse of the lipid bilayer and a termination of the lipid vesicle’s existence (to preserve the most energetically favourable state), the only protective mechanism is the pores’ creation, and/or the emergence (evolution) of an active pumping mechanism across the membrane. This also supports Premise 2, in that no active pumping mechanism would be required in membranes that are permeable to protons (Mulkidjanian et al. 2012).
Electrochemical Gradient
The available free energy \( \Delta {G}_{ech}^{H^{+}} \) associated with the formation of the electrochemical proton gradient across the membrane of the lipid vesicle was calculated with the equation formulated by Mitchell (1961):
where F is the Faraday constant (F = 9.65 × 104 C.mol−1), ∆ψ is the transmembrane electric potential difference caused by the release of protons into the interior of the lipid vesicle through the flip-flop mechanism, R is the gas constant (R = 8.314 J.K−1.mol−1), T is the temperature in the range of 0–100 °C, and ∆pH is the transmembrane pH difference.
The transmembrane potential ∆ψ was calculated using the Nernst equation:
where the transmembrane pH difference ∆pH is defined as follows:
where [H+]in and [H+]out are the proton concentration inside and outside the vesicle, respectively. Further:
where [H+]flip-flop is the concentration of protons released into the lipid vesicle through the flip-flop mechanism of the incorporated lipids.
The incorporation of the new lipid molecules into the vesicle results in the elevated concentration of protons inside the vesicle. The maximal theoretical efficiency of the protons’ transportation via the flip-flop mechanism is 25 %; thus, [H+]flip-flop = [H+]inc/4. However, as we only count lipids from the outer side of the membrane, the final theoretical concentration of protons transferred into the lipid vesicle is:
Thus, theoretically only 12.5 % of the new incorporated lipids release the proton into the vesicle ([H+]flip-flop), which is in good agreement with the experimental findings of Chen and Szostak (2004) of ≈ 12 %.
We simulated the pH decrease inside the vesicle that is dependent upon the number of incorporated lipid molecules. Their number was in the range of 1 – Nlip 100 nm, where Nlip 100 nm is the maximum number of lipids corresponding to the lipid vesicle 100 nm in size. Therefore, the maximum value (Nlip 100 nm) demonstrates the fusion of two vesicles 100 nm in size. All simulations were performed in Matlab R2011b.
Before the incorporation of the new lipid molecules into the vesicle, the overall proton concentration outside (in the proximity of the membrane surface) the lipid bilayer (membrane) was equal to the proton concentration inside the vesicle, and equal to the proton concentration due to the initial pH ([H+]pH):
The initial pH value (5.5) was chosen to reflect the hot, acidic environment during early life on Earth (Perez-Jimenez et al. 2011).
Osmotic Pressure
The osmotic pressure associated with the flow of water molecules into the lipid vesicle with the membrane impermeable to protons is defined as follows:
where [H+]flip-flop is the concentration of protons transferred into the lipid vesicle via the flip-flop mechanism of incorporated lipid molecules (units [mol/L]), R is the gas constant (R = 8.314 J.K−1.mol−1), and T is the temperature in the range of 0–100 °C.
Results and Discussion
The pH decrease inside the lipid vesicle (defined in Simulation and Calculation Methods section), caused by the incorporation of new lipids into the vesicle (fusion with micelle), is presented in Fig. 1. The size of the initial lipid vesicle is 100 nm. The maximum pH drop from the initial value of 5.5 down to the value of 2.4 demonstrates the fusion of the two identical vesicles 100 nm in size—the maximum number of incorporated lipids is the same as the number of lipids in the vesicle 100 nm in size.
Figure 2 shows the difference in the pH of the lipid vesicle’s interior and exterior, after incorporation of the new lipid molecules. The proton release is due to the lipids’ flip-flop mechanism in the membrane. The shape of the curve is in agreement with Morowitz’s (1992) findings, which assert that merely the addition of a single proton within a small vesicle results in significant pH changes. In our simulations, the release of one proton into the lipid vesicle resulted in a change of pH in ≈ 0.01 units.
The broadly accepted explanation for the relative impermeability of the lipid membranes to protons, as well as to ions, is the fact that protons from a high-dielectric aqueous phase have to travel through the low-dielectric membrane interior composed of hydrocarbon chains (Parsegian 1969). The energy required to overcome this barrier is in the range of 100–300 kJ/mol for inorganic monovalent cations. In Fig. 3, the free Gibbs energy associated with the creation of the pH gradient (Eq. 1) across the lipid bilayer (ΔGgrad) is presented. Due to Chen and Szostak’s (2004) findings, the conversion of micelles to vesicles is exergonic (ΔG < 0), which is in agreement with our results. For our simulation parameters, the values of free energy are in the range of 0.01–44.2 kJ/mol, which is still insufficient for the forced transfer across the membrane. On the other hand, it is well known that protons permeate the lipid bilayer several orders of magnitude faster than other small positive ions. One of the theories supposed the formation of membrane-spanning single-file water wires providing a matrix along which the protons can pass through the barrier (Tepper and Voth 2005). Marrink et al. (1996) calculated the free energy necessary for the formation of such water wire as 26 kcal/mol (108.8 kJ/mol). Similarly, in a recent theoretical study, the free energy of lipid bilayer pore formation (passive transport) was estimated at 10–24 kcal/mol (41.9–100.5 kJ/mol) (Leontiadou et al. 2004), which overlaps with our results.
Recent study estimated the bending stiffness (kc) of the lipid bilayer at 18k B T and 57k B T for the liquid and gel phases, respectively (Picas et al. 2012). With our parameters of the lipid vesicle, it is represented by the following values: liquid phase (kc,liq) 6.785 × 10−20 – 9.269 × 10−20 J; gel phase (kc,gel) 2.15 × 10−19 – 2.94 × 10−19 J. Simulated data of the free energy associated with the creation of the electrochemical proton gradient and osmotic pressure of the lipid bilayer membrane are as follows—the increase of energy corresponds to the incorporation of a higher number of new lipid molecules into the vesicle 100 nm in size, where the maximum value demonstrates the fusion of two identical vesicles 100 nm in size:
-
Free energy of proton electrochemical gradient: ΔGgrad = 1.7 × 10−23 − 7.3 × 10−16J
-
Free energy of osmotic pressure: ΔGΠ = 4.7 × 10−22 − 5.2 × 10−17J
-
Sum of free energy: ΔGsum = ΔGgrad + ΔGΠ = 4.9 × 10−22 − 7.9 × 10−16J (Fig. 4)
These values suggest that the energy associated with the emergence of the electrochemical proton gradient itself is sufficient to overcome the bending stiffness of the lipid bilayer. Moreover, it is multiplied by the energy associated with osmotic pressure. For the lipid vesicle 100 nm in size the critical number of incorporated lipids is in the order of hundreds molecules, which is characterised by pH changes in 0.68 units for the liquid phase and 0,85 units for the gel phase, respectively (for temperature T = 40 °C, Fig. 4).
Conclusions
With the support of simple simulations, we have demonstrated that the emergence of a pH gradient across the membrane of early lipid vesicles (as a consequence of vesicle growth after the incorporation of new lipid molecules), can lead to forced proton release from the vesicle through the pores’ formation (passive transport), and/or evolution of an active transport mechanism across the lipid bilayer, as a consequence of early lipid vesicles natural selection from physico-chemical point of view, capable of further Darwinian evolution.
References
Adamala K, Szostak JW (2013) Competition between model protocells driven by an encapsulated catalyst. Nat Chem 5:495–501. doi:10.1038/nchem.1650
Berclaz N, Muller M, Walde P, Luisi PL (2001) Growth and transformation of vesicles studied by ferritin labeling and cryotransmission electron microscopy. J Phys Chem B 105:1056–1064. doi:10.1021/jp001298i
Budin I, Szostak JW (2011) Physical effects underlying the transition from primitive to modern cell membranes. Proc Natl Acad Sci U S A 108:5249–5254. doi:10.1073/pnas.1100498108
Chen IA, Szostak JW (2004) Membrane growth can generate a transmembrane pH gradient in fatty acid vesicles. Proc Natl Acad Sci U S A 101:7965–7970. doi:10.1073/pnas.0308045101
Chen IA, Roberts R, Szostak JW (2004) The emergence of competition between model protocells. Science 305:1474–1476. doi:10.1126/science.1100757
Cheng Z, Luisi PL (2003) Coexistence and mutual competetion of vesicles with different size distributions. J Phys Chem B 107:10940–10945. doi:10.1021/jp034456p
Chyba C, Sagan C (1992) Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventor for the origins of life. Nature 355:125–132. doi:10.1038/355125a0
Deamer DW (1985) Boundary structures are formed by organic components of the Murchison carbonaceous chondrite. Nature 317:792–794. doi:10.1038/317792a0
Groen J, Deamer DW, Kros A, Ehrenfreund P (2012) Polycyclic aromatic hydrocarbons as plausible prebiotic membrane components. Orig Life Evol Biosph 42:295–306. doi:10.1007/s11084-012-9292-3
Hanczyz MM, Fujikawa SM, Szostak JW (2003) Experimental models of primitive cellular compartmentalization: Encapsulation, growth, and division. Science 302:618–622. doi:10.1126/science.1089904
Leontiadou H, Mark AE, Marrink SJ (2004) Molecular dynamics simulations of hydrophilic pores in lipid bilayers. Biophys J 86:2156–2164. doi:10.1016/S0006-3495(04)74275-7
Mansy SS (2010) Membrane transport in primitive cells. Cold Spring Harb Perspect Biol 2:a002188. doi:10.1101/cshperspect.a002188
Marrink SJ, Jahnig F, Berendsen HJC (1996) Proton transport across transient single-file water pores in a lipid membrane studied by molecular dynamics simulations. Biophys J 71:632–647. doi:10.1016/S0006-3495(96)79264-0
Maurer SE, Deamer DW, Boncella JM, Monnard PA (2009) Chemical evolution of amphiphiles: Glycerol monoaclyl derivatives stabilize plausible prebiotic membranes. Astrobiology 9:979–987. doi:10.1089/ast.2009.0384
Mavelli F, Ruiz-Mirazo K (2010) Enviroment: a computational platform to stochastically simulate reacting and self-reproducing lipid compartments. Phys Biol 7:036002. doi:10.1088/1478-3975/7/3/036002
McCollom TM, Seewald JS (2007) Abiotic synthesis of organic compounds in deep-sea hydrothermal environments. Chem Rev 107:382–401. doi:10.1021/cr0503660
Mitchell P (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191:144–148. doi:10.1038/191144a0
Morowitz HJ (1992) Beginnings of cellular life. Metabolism recapitulates biogenesis. Yale University Press, New Haven
Mulkidjanian AY, Bychkov AY, Dibrova DV, Galperin MY, Koonin EV (2012) Origin of first cells at terrestrial, anoxic geothermal fields. Proc Natl Acad Sci U S A 109:E821–E830. doi:10.1073/pnas.1117774109
Munteanu A, Attolini CSO, Rasmussen S, Ziock H, Sole RV (2007) Generic darwinian selection in catalytic protocell assemblies. Philos Trans R Soc Lond Ser B Biol Sci 362:1847–1855. doi:10.1098/rstb.2007.2077
Namani T, Deamer DW (2008) Stability of model membranes in extreme environments. Orig Life Evol Biosph 38:329–341. doi:10.1007/s11084-008-9131-8
Parsegian A (1969) Energy of an ion crossing a low dielectric membrane: Solutions to four relevant electrostatic problems. Nature 221:844–846. doi:10.1038/221844a0
Perez-Jimenez R, Ingles-Prieto A, Zhao ZM, et al. (2011) Single-molecule paleoenzymology probes the chemistry of resurrected enzymes. Nat Struct Mol Biol 18:592–596. doi:10.1038/nsmb.2020
Picas L, Rico F, Scheuring S (2012) Direct measurement of the mechanical properties of lipid phases in supported bilayers. Biophys J 102:L01–L03. doi:10.1016/j.bpj.2011.11.4001
Pohl EE, Peterson U, Sun J, Pohl P (2000) Changes of intrinsic membrane potentials induced by flip-flop of long-chain fatty acids. Biochemistry 39:1834–1839. doi:10.1021/bi9919549
Rushdi AI, Simoneit BR (2001) Lipid formation by aqueous Fischer-Tropsch-Type synthesis over temperature range of 100 to 400 °C. Orig Life Evol Biosph 31:103–118. doi:10.1023/A:1006702503954
Sojo V, Pomiankowski A, Lane N (2014) A bioenergetic basis for membrane divergence in Archea and Bacteria. PLoS Biol 12(8):e1001926. doi:10.1371/journal.pbio.1001926
Tepper HL, Voth GA (2005) Protons may leak through pure lipid bilayers via a concerted mechanism. Biophys J 88:3095–3108. doi:10.1529/biophysj.104.056184
Acknowledgments
This work results within the collaboration of the COST Action TD 1308.
Author information
Authors and Affiliations
Corresponding author
Additional information
Paper presented at the conference: Habitability in the Universe: From the Early Earth to Exoplanets 22–27 March 2015, Porto, Portugal
Rights and permissions
About this article
Cite this article
Strbak, O., Kanuchova, Z. & Krafcik, A. Proton Gradients as a Key Physical Factor in the Evolution of the Forced Transport Mechanism Across the Lipid Membrane. Orig Life Evol Biosph 46, 523–531 (2016). https://doi.org/10.1007/s11084-016-9496-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-016-9496-z