Abstract
Here we summarize the main results of our latest investigation on the spontaneous encapsulation of proteins (ferritin) and ribosomes inside lipid vesicles. We show that when vesicles form in a solution containing some macromolecules (even at low concentration), in contrast to the expectations, a few but measurable number of vesicles is able to capture a very high number of solutes, up to 60 times the external concentration. We also show preliminary evidences on the encapsulation of additional solutes (ribo-peptidic complexes, fluorescent proteins and enzymes), and shortly present our current approach aimed at exploiting this phenomenon. In particular, we would like to reveal how the formation of compartments can trigger effective intra-vesicle reactions starting from diluted solutions. Although the mechanistic details for this phenomenon are still missing, we claim that these new evidences are highly relevant for the origin of the first functional cells in primitive times.
Similar content being viewed by others
The Formation of Primitive Cells
One of the key and still unanswered questions in origin of life research is the formation of primitive cells. There is indeed a gap between studies of the early chemical evolution and the biological pathways starting from the last universal common ancestor. In particular, since it is well accepted that semi-permeable membrane vesicles represent the most plausible precursors of cells (Deamer and Dworkin 2005), one open question would be the time at which these compartments came into the picture as hosts for the first forms of metabolism.
For the “metabolism first” and “replication first” scenarios, we are faced with a problem: how could all the molecules - which were firstly developed in solution - become simultaneously incorporated, later on, into a closed compartmentalised structure?
Alternatively, in order to let the first forms of metabolism arise inside closed compartments, and become more complex later, it is necessary that primitive membranes have an efficient, directional, and preferably controlled material exchange with the environment. But simple vesicular structures lack control of membrane permeability. So, either way, we are facing a conceptual conundrum.
Starting from our previous considerations on the encapsulation of solutes inside lipid vesicles (Luisi 2006), we recently started a direct investigation of this process, aimed at understanding the role of physical self-organization in the onset of cell-like particles. We carried out a systematic investigation based on cryo-transmission electron microscopy (cryo-TEM) of solute-containing vesicles (Luisi et al. 2010; Souza et al. 2011), and we are currently expanding these studies to include other solutes that cannot be analyzed by cryo-TEM. With surprise, we discovered an unexpected phenomenon that might help to clarify some aspects of the above-mentioned conundrum.
In this contribution, we summarize our recent studies on the spontaneous assembly of primitive cell-like structures, by using lipid vesicles (liposomes) as cellular models. We show that lipid vesicles can spontaneously capture a very high number of macromolecular solutes even when formed in diluted solutions, overcoming one of the major problems in prebiotic chemistry, i.e. the expected low concentration of solutes. As a result of this intriguing behavior, these solute-rich vesicles could be able to facilitate and support the emergence of a cellular metabolism (Luisi 2012), thanks to the molecular richness in their aqueous core. We believe that our investigation reveals an important new concept for the origin of cells and more in general for self-organizing systems.
The Encapsulation of Ferritin and Ribosomes Inside Liposomes
Our experimental model consists in the formation of lipid vesicles in a solution of a macromolecular solute. Vesicles, formed by 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine (POPC) or POPC/cholesterol mixtures, were formed by two classical methods, namely thin film hydration (with or without extrusion to a definite size) and ethanol injection. The solutes present in the solution can be entrapped within the lipid vesicles at the moment of their formation. Due to the impermeability of lipid membranes to such large molecules, the analysis of the liposome contents provides realistic insights in the physics of solute encapsulation. In order to detect the molecular content of each vesicle, at the level of a single molecule, it is necessary to use solutes that can be individually visualized by cryo-TEM, which is the best method for imaging lipid vesicles. Based on our previous studies of the ‘matrix effect’ (Berclaz et al. 2001a,b), we first used ferritin (Luisi et al. 2010), a 12.5 nm large water-soluble protein which consists of 24 subunits enclosing a spherical cavity where iron is stored in form of hydrous ferric oxide phosphate (ca. 4,000 iron atoms/ferritin). Thanks to its iron core, ferritin molecules can be easily detected by cryo-TEM and counted individually. Next, we also used ribosomes (Souza et al. 2011), that can be visualized by cryo-TEM even if they miss heavy metal atoms. The choice of entrapping ribosomes was because of their relevance as key compounds for protein synthesis and therefore for the development of primitive functional cells.
The starting point of our discussion is the formulation of the simplest hypothesis for solute entrapment inside vesicles. When a vesicle of volume V is formed in a solution containing a certain solute with concentration C 0, this process can be considered as equivalent to sampling the solution. It is then expected that, on average, a vesicle will contain N 0 molecules, where N 0 = N A V C 0 (N A being the Avogadro number). Clearly, not all vesicles will contain N 0 molecules, because of stochastic fluctuation of the solute concentration. These variations around the average value N 0 are described by a Poisson distribution, which gives the probability of finding a vesicle (with volume V) containing N molecules instead of N 0. For example, consider a 100 nm (diameter) vesicle that forms in a 9.5 μM ferritin solution. In these condition, N 0 = 3. According to the Poisson distribution, it is expected that about 5 % of vesicles are empty, most of the vesicles will contain 3 ferritin molecules, and the probability of finding a vesicle with many more ferritin molecules (let us say, 10 or more) will be very low. A bell-shaped curve will describe the solute occupancy distribution.
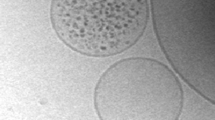
These being the expectations let us see what we observed. As shown in Fig. 1a–b, both for ferritin and ribosomes, the direct observation of liposome samples after solute entrapment revealed an unexpected outcome. In particular, the first important observation was that the majority of vesicles (>80 %) were empty. The frequency of solute-filled vesicles decreases as the number N of entrapped molecules increases, and striking enough, we observed that about 0.1–1 % of vesicles contained a very high number of solutes (N > 20, up to ca. 300), in clear contrast to the prediction of the Poisson law. As pictorially evident in Fig. 1a–b, the outcome of the spontaneous entrapment of these macromolecules inside lipid vesicles can be emphatically described as ‘all-or-nothing’ pattern, rather than by a bell-shaped solute occupancy distribution.
Entrapment of ferritin (a) and ribosomes (b) inside lipid vesicles, as shown by cryo-TEM imaging (size bar: 100 nm). Note that both ferritin and ribosomes, although present at high concentration inside the vesicles, are not aggregated. Panel (c): Ferritin occupancy distribution profiles, obtained for 4 different experiments, where ferritin concentration (C 0) was 4 (diamonds), 8 (circles), 16 (squares) or 32 μM (triangles). Filled symbols: experimental distribution; empty symbols connected by a line: expected Poisson distribution. Similar results have been obtained for ribosome entrapment. Note the bell-shaped Poisson curve, centered on the expected number of entrapped ferritin (N 0) in vesicles of diameter 100 nm, versus the monotonally decreasing experimental curve. Panel (d) shows the same experimental data presented in panel (c) but rendered in a bi-logarithmic plot. This makes evident that the ferritin occupancy distribution follows a power law. Panels (a,c,d) reproduced from Luisi et al. (2010) with permission from Springer. Panel (b) reproduced from Souza et al. (2011) with permission from Springer
Figure 1c shows the experimental (versus Poisson) distribution of vesicles containing ferritin, obtained after directly counting the number of ferritin molecules inside 7,700 vesicles, prepared from different ferritin solution (C 0 from 4 to 32 μM). Similar results have been obtained in the case of ribosome containing vesicles (ca. 400 vesicles; C 0 ranging from 0.48 to 8 μM). It can be seen how the experimental distributions deviate substantially from the Poisson curves. In particular, the frequency of vesicles containing 0, 1, 2, … N solutes decreases monotonically, but very slowly. Thanks to this specific patter, it turns out that the existence of ‘super-filled’ vesicles is not as improbable as calculated by the Poisson curve. For example, the Poisson probability of finding a 100-nm vesicle containing 50 ferritin molecules, when C 0 = 8 μM, is about 10−46, whereas we found that actually we can find one such vesicle out of 1,000 vesicles (10−3). If the data of Fig. 1c are plotted in a bi-logarithmic plot, as shown in Fig. 1d, it becomes evident that the solute occupancy distribution seems to follow a power law profile. We are currently investigating the generative mechanism of this power law distribution, and its connection with the mechanism of vesicle formation and solute capture (Mavelli et al., manuscript in preparation).
But there is a second and more important consequence of deviation from the Poisson law in favor of the power law. In fact, despite the fact that most of the vesicles are empty, the filled vesicles – actually ‘super-filled’ – contain a number N of solutes that exceed the expected value N 0. This means that the local (intra-vesicle) solute concentration C is higher than the bulk concentration C 0. In other words, these vesicles have actively recruited solutes during their formation, bringing about a concentration enhancement of factors up to about 60. The local concentration then might rise from a rather diluted value, e.g., 10 μM, to 0.6 mM. By considering the volume occupied by the solutes confined in the tiny intra-vesicle lumen, it has been estimated that in many cases – especially for the smaller vesicles – these values correspond to crowding concentration, typical of living cells.
A more detailed discussion of these data can be found in the original articles (Luisi et al. 2010; Souza et al. 2011), as well as a proposal for the mechanism underlying the effect of solute ‘super-concentration’, up to crowding conditions. Here it is useful to add a short comment on the fact that such anomalous behavior helps explain in a rather clear way another set of experimental data obtained by encapsulating the complex transcription/translation machinery (about 80 macromolecular compounds, plus amino acids, nucleotides, etc.) inside 200 nm vesicles (Souza et al. 2009). In order to explain the success of intra-vesicle protein production (that was against the theoretical prediction of simultaneous co-entrapment of 80 different macromolecules inside such small vesicles) we made the hypothesis that the local concentration of these molecules should have reached an enhancement factor of about 20. If solutes are encapsulated according to a power law rather than a Poisson law, this enhancement factor becomes realistic (for a stochastic simulation, see Lazzerini-Ospri et al. 2012) and would explain how 200 nm vesicles can indeed entrap a complex molecular ‘soup’ and concentrate these compounds inside. Cryo-TEM images of vesicles formed in this way were also published (Souza et al. 2011).
The Case of Ribo-Peptidic Complexes
Having shown the intriguing encapsulation pattern of ferritin and ribosomes, we reasoned that an additional proof of concept on the relevance of this phenomenon for the origin of life, would consist in the extension of these results to the encapsulation of ‘primitive’ compounds. In the past years, we investigated the formation of very simple ribo-peptidic complexes, which roughly resemble ribosomes (D’Aguanno 2009). These are ionic complexes formed between ribonucleic acids, like tRNA or rRNA, mixed with polycations, such as poly-L-lysine or poly-L-arginine. Since these complexes can be visualized by cryo-TEM, we checked whether they can be also entrapped inside lipid vesicles formed in situ as happens for ferritin and ribosomes.
Firstly, we optimized the method of complex preparation by varying the concentration and the molar ratio of the compounds used (20 nt RNA, and 7.5 kDa poly-L-arginine), in order to get a population of narrowly distributed particles, with a diameter of about 5 nm (Fig. 2a, see details in the figure caption). Then we used these particles, which mimic primitive ribo-peptide complexes, for an experiment of solute encapsulation. The observation of a few vesicles quite filled with the complexes, together with a large number of empty vesicles (Fig. 2b), agrees with previous results obtained with ferritin and ribosomes. This experiment, although missing a quantitative statistical analysis, reveals that a general encapsulation mechanism operates to produce the ‘super-filled’ vesicles, and that it is independent of evolved macromolecular sequences, being also observed for very simple molecular moieties.
Ribo-peptidic complexes (a) and their encapsulation inside lipid vesicles (b), pre-stained with phosphotungstic acid, as shown by cryo-TEM imaging, (size bar: 100 nm). Ribo-peptidic complexes were prepared by mixing 15 μM of 20-nt double stranded RNA (i.e., 40 residues) with 6.6 μM of 7.5 kDa poly-L-arginine (39 residues long) in 20 mM bicine (sodium salt, pH 8.5), corresponding to a molar fraction of positive charges equal to 0.3. The suspension of ribo-peptidic particles, shown in (a) was then incubated with 1 % phosphotungstic acid (after adjustement of the stock solution pH to 7.6), and later used to prepare POPC vesicles (b). Vesicles were prepared, manipulated, and imaged as reported in Luisi et al. (2010) and Souza et al. (2011)
Work in Progress
Until now our research has been focused on sub-micron vesicles and on solutes that can be visualized by cryoTEM, in order to demonstrate the deviation of the encapsulation process from the expected Poisson law and verify by direct imaging the existence of ‘super-filled’ vesicles. Currently we are working on expanding these initial studies to larger vesicles and fluorescent solutes. This will allow us to use standard fluorescence microscopy (confocal) to monitor not only static molecules like ferritin, but to follow dynamical processes as single- or multi-enzyme processes. The only requisite is that the solutes themselves or the product of the reaction they catalyse must be fluorescent. For this aim we are currently involved in investigating the encapsulation of proteins and other solutes in micrometer sized vesicles formed by film hydration (on glass beads) or ethanol injection.
Preliminary results indicate that a wide range of solutes actually behave as shown for ferritin, ribosomes and ribo-peptidic complexes: most vesicles turn out to be empty, and a few vesicles contain a number of solute molecules much higher than expected. Due to the larger vesicle size, the expected number N 0 of entrapped solute is now high (for vesicle diameter of 1 μm and C 0 = 9.5 μM, N 0 ~ 24,000). Nevertheless, preliminary data (see Fig. 3a-b), obtained for fluorescently labeled albumin and dextranes, phycoerythrin, allophycocyanin, and for the enzymes carbonic anhydrases and protease K, all show that it is possible to observe – although in a few vesicles - local concentration enhancement factors from 3 to 4 (most frequent, see Fig. 3c-d) to 10–15 (rarely) (D’Aguanno et al., work in progress). According to these first data, it appears that by increasing vesicle size, the magnitude of the ‘super-concentration’ effect is slightly reduced when compared with sub-micron vesicles, suggesting a physical role related to the surface-to-volume ratio. We have not yet investigated the case of giant vesicles (diameter above 5 μm), but an interesting report from the group of Keating (Dominak and Keating 2007) shows that similar (but weaker) effects still occur in giant vesicles.
Entrapment of fluorescent proteins and dextranes inside lipid vesicles. Fluorescence micrographs, obtained by confocal laser scanning microscopy, showing (a) 920 nm POPC vesicle containing fluorescein-labelled bovine serum albumine (BSA-FITC); and (b) 1,080 nm POPC vesicle containing phycoerythrin (PE). Shortly, POPC vesicles were prepared by hydrating POPC films deposited on 10 glass beads (2 mm diameter, 16 nmoles POPC/bead) with a solution (200 μL) of 1 μM BSA-FITC (or PE). After vesicle formation, the sample has been visualized without any additional treatment. Free solutes are present outside vesicles. Panels (c) and (d) show the quantitation of fluorescence along a line crossing the vesicle in panels (a) and (b), respectively. The fluorescence contribution of non-entrapped BSA-FITC (or PE) accounts for 18 (or 22) fluorescence a.u., whereas the internal BSA-FITC (or PE) accounts for about 96 (or 89) fluorescence a.u. In this case, the solutes were concentrated by a factor 5.3 (or 4.0). In other, rarer cases it was possible to observe concentration factors of about 10–15
Most interestingly, the possibility of fluorescence detection of super-filled vesicles triggered us to simulate primitive events of spontaneous intra-vesicle solute concentration by the protein synthesis model (specifically, green fluorescent protein synthesis). Vesicles are formed in situ in sluggishly reacting, diluted transcription/translation machinery – a model of an inefficient metabolic network. If vesicles were able to concentrate in their core all the molecules needed to synthesize protein, so that their internal concentrations become 10–20 times higher than in the bulk, an efficient protein synthesis could be observed only inside liposomes, whereas almost no reaction should occur in the outer environment (Stano et al., work in progress). This would demonstrate in a clear-cut way the active role of compartments for triggering an efficient metabolism starting from diluted solution, and emphasize the role of confinement and local concentration in the early steps toward the first living cells.
Concluding Remarks
In conclusion, we believe that we have brought new evidences on the question of the origin of functional cells in primitive times. We have observed, by cryo-TEM imaging, that when vesicles form spontaneously in a solution of diluted solutes (of different chemical nature and molecular weight), whereas most of the vesicles are empty, few of them are able to entrap a very high number of solutes, so that a very high internal concentration of that solute is reached (possibly in the crowding regime), against the entropic expectations. This shows that vesicles might actively favor the onset of a compartmentalized metabolism on the basis of purely physico-chemical forces. Although the mechanistic details are still unclear, experimental evidences clearly point to the involvement of surface effects and cooperative behavior based on weak solute/solute and solute/membrane interactions. Finally, it should be recalled that the spontaneous formation of cell-like structures, based on the observation reported here, can pave the way to direct investigations in simulated diluted primitive conditions, so that the consequences on reaction efficiencies can be experimental examined.
References
Berclaz N, Blöchliger E, Müller M, Luisi PL (2001a) Matrix effect of vesicle formation as investigated by cryotransmission electron microscopy. J Phys Chem B 105:1065–1071
Berclaz N, Müller M, Walde P, Luisi PL (2001b) Growth and transformation of vesicles studied by ferritin labeling and cryotransmission electron microscopy. J Phys Chem B 105:1056–1064
D’Aguanno E (2009) Studi su modelli prebiotici di ribosomi (Master Thesis). University of Roma Tre, Rome
Deamer DW, Dworkin JP (2005) Chemistry and physics of primitive membranes. Top Curr Chem 259:1–27
Dominak LM, Keating CD (2007) Polymer encapsulation within giant lipid vesicles. Langmuir 23:7148–7154
Lazzerini-Ospri L, Stano P, Luisi PL, Marangoni R (2012) Characterization of the emergent properties of a synthetic quasi-cellular system. BMC Bioinforma 13(Suppl 4):S9
Luisi PL (2006) The Emergence of Life: from Chemical Origin to Synthetic Biology. Cambridge University Press, Cambridge
Luisi PL (2012) On the emergence of metabolism. Helv Chim Acta, in press
Luisi PL, Allegretti M, Souza T, Steineger F, Fahr A, Stano P (2010) Spontaneous protein crowding in liposomes: A new vista for the origin of cellular metabolism. ChemBioChem 11:1989–1992
Souza T, Stano P, Luisi PL (2009) The minimal size of liposome-based model cells brings about a remarkably enhanced entrapment and protein synthesis. ChemBioChem 10:1056–1063
Souza T, Steiniger F, Stano P, Fahr A, Luisi PL (2011) Spontaneous crowding of ribosomes and proteins inside vesicles: A possible mechanism for the origin of cell metabolism. ChemBioChem 12:2325–2330
Acknowledgements
This paper shortly summarizes our contribution at the Third Edition of the “Open Questions on Origins of Life” workshop (Leicester, 1–4 May 2012). We gratefully thank Prof. Dagmar Fischer (Institut für Pharmazie, Friedrich Schiller Universität Jena) for providing short RNA strands. T. P. S. was supported by the Alexander von Humboldt Foundation as a post-doctoral fellow. This work was funded by the SYNTHCELLS project (Approaches to the Bioengineering of Synthetic Minimal Cells, EU FP6 043359), HFSP (RGP0033/2007-C), ASI (I/015/07/0), and PRIN2008 (2008FY7J4). It was also developed within the COST Systems Chemistry action (CM0703).
Author information
Authors and Affiliations
Corresponding author
Additional information
Tereza Pereira de Souza and Pasquale Stano contributed equally
Rights and permissions
About this article
Cite this article
de Souza, T.P., Stano, P., Steiniger, F. et al. Encapsulation of Ferritin, Ribosomes, and Ribo-Peptidic Complexes Inside Liposomes: Insights Into the Origin of Metabolism. Orig Life Evol Biosph 42, 421–428 (2012). https://doi.org/10.1007/s11084-012-9303-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-012-9303-4