Abstract
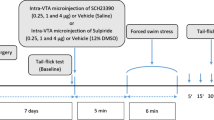
Sensitization to psychostimulant drugs, as well as morphine, subjected to cross-sensitization with stress. The development of morphine sensitization is associated with enhancements in dopamine overflow in the Nucleus accumbens (NAc). This study aimed to examine the role of accumbal D1/D2-like dopamine receptors in restraint stress (RS) induced sensitization to morphine antinociceptive effects. Adult male Wistar rats weighing 220–250 g underwent stereotaxic surgery. Two stainless steel guide cannulae were bilaterally implanted, 1 mm above the NAc injection site. Different solutions of SCH-23390, as a D1-like receptor antagonist or sulpiride, as a D2-like receptor antagonist, were microinjected into the NAc five min before exposure to RS. Restraint stress lasted for 3 h, 10 min after RS termination; animals received a subcutaneous injection of morphine (1 mg/kg) for 3 consecutive days. The procedure was followed by a 5-day drug and/or stress-free period. After that, on the 9th day, the nociceptive response was evaluated by the tail-flick test. The results revealed that intra-NAc administration of D1/D2-like dopamine receptor antagonists, SCH-23390 or sulpiride, respectively, blocked morphine sensitization-induced by RS and morphine co-administration in rats for three consecutive days. This work provides new insight into the determinant role of accumbal dopamine receptors in morphine sensitization produced by RS-morphine co-administration.







Similar content being viewed by others
References
Phillips TJ (1997) Behavior genetics of drug sensitization. Crit Rev Neurobiol 11:21–33. https://doi.org/10.1615/CritRevNeurobiol.v11.i1.20
Vezina P, Leyton M (2009) Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology 56:160–168. https://doi.org/10.1016/j.neuropharm.2008.06.070
Reisi Z, Bani-Ardalan M, Zarepour L, Haghparast A (2014) Involvement of D1/D2 dopamine receptors within the nucleus accumbens and ventral tegmental area in the development of sensitization to antinociceptive effect of morphine. Pharmacol Biochem Behav 118:16–21. https://doi.org/10.1016/j.pbb.2013.12.023
Lv Y, Rong Hu R, Jing M, Yun Zhao T, Wu N, Song R, Li J, Hu G (2019) Selective dopamine D3 receptor antagonist YQA14 inhibits morphine-induced behavioral sensitization in wild type, but not in dopamine D3 receptor knockout mice. Acta Pharmacol Sin 40:583–588. https://doi.org/10.1038/s41401-018-0153-0
Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci 29:565–598. https://doi.org/10.1146/annurev.neuro.29.051605.113009
Cheng YC, Tsai RY, Sung YT, Chen IJ, Tu TY, Mao YY, Wong CS (2019) Melatonin regulation of transcription in the reversal of morphine tolerance: microarray analysis of differential gene expression. Int J Mol Med 43:791–806. https://doi.org/10.3892/ijmm.2018.4030
T.J. Zhang, Y. Qiu, Z. Hua. (2019). The Emerging Perspective of Morphine Tolerance: MicroRNAs, Pain Res Manag 2019. https://doi.org/https://doi.org/10.1155/2019/9432965.
Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stöckl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A (2007) Explaining the escalation of drug use in substance dependence: Models and appropriate animal laboratory tests. Pharmacology 80:65–119. https://doi.org/10.1159/000103923
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 85:5274–5278. https://doi.org/10.1073/pnas.85.14.5274
Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D (2004) Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology 47:227–241. https://doi.org/10.1016/j.neuropharm.2004.06.032
Covey DP, Bunner KD, Schuweiler DR, Cheer JF, Garris PA (2016) Amphetamine elevates nucleus accumbens dopamine via an action potential-dependent mechanism that is modulated by endocannabinoids. Eur J Neurosci 43:1661–1673. https://doi.org/10.1111/ejn.13248
Corre J, Van Zessen R, Loureiro M, Patriarchi T, Tian L, Pascoli V, Lüscher C (2018) Dopamine neurons projecting to medial shell of the nucleus accumbens drive heroin reinforcement. Elife 7:e39945
Tjon GHK, De Vries TJ, Ronken E, Hogenboom F, Wardeh G, Mulder AH, Schoffelmeer ANM (1994) Repeated and chronic morphine administration causes differential long-lasting changes in dopaminergic neurotransmission in rat striatum without changing its δ- and κ-opioid receptor regulation. Eur J Pharmacol 252:205–212. https://doi.org/10.1016/0014-2999(94)90598-3
Beyer CE, Steketee JD (2002) Cocaine sensitization: modulation by dopamine D2 receptors. Cereb Cortex 12:526–535. https://doi.org/10.1093/cercor/12.5.526
Charmchi E, Zendehdel M, Haghparast A (2016) The effect of forced swim stress on morphine sensitization: Involvement of D1/D2-like dopamine receptors within the nucleus accumbens. Prog. Neuro-Psychopharmacol Biol Psychiatry 70:92–99. https://doi.org/10.1016/j.pnpbp.2016.05.006
Shaham Y, Kelsey JE, Stewart J (1995) Erratum: Temporal factors in the effect of restraint stress on morphine-induced behavioral sensitization in the rat (Psychopharmacology (1995) 117 (102–109)). Psychopharmacology (Berl). 118:480
Booij L, Welfeld K, Leyton M, Dagher A, Boileau I, Sibon I, Baker GB, Diksic M, Soucy JP, Pruessner JC (2016) Dopamine cross-sensitization between psychostimulant drugs and stress in healthy male volunteers. Transl Psychiatry. 6:e740–e740
Holly EN, Debold JF, Miczek KA (2015) Increased mesocorticolimbic dopamine during acute and repeated social defeat stress: Modulation by corticotropin releasing factor receptors in the ventral tegmental area. Psychopharmacology (Berl) 232:4469–4479. https://doi.org/10.1007/s00213-015-4082-z
Imperato A, Puglisi-Allegra S, Casolini P, Zocchi A, Angelucci L (1989) Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. Eur J Pharmacol 165:337–338. https://doi.org/10.1016/0014-2999(89)90735-8
Moore H, Rose HJ, Grace AA (2001) Chronic cold stress reduces the spontaneous activity of ventral tegmental dopamine neurons. Neuropsychopharmacology 24:410–419. https://doi.org/10.1016/S0893-133X(00)00188-3
Y. Razavi, S. Karimi, M. Bani-Ardalan, A. Haghparast (2014) Chemical stimulation of the lateral hypothalamus potentiated the sensitization to morphine in rats: Involvement of orexin-1 receptor in the ventral tegmental area. EXCLI J 13: 1120–1130. https://doi.org/https://doi.org/10.17877/DE290R-7236.
Abad ATK, Miladi-Gorji H, Bigdeli I (2016) Effects of swimming exercise on morphine-induced reward and behavioral sensitization in maternally-separated rat pups in the conditioned place preference procedure. Neurosci Lett 631:79–84. https://doi.org/10.1016/j.neulet.2016.08.011
Pang G, Wu X, Tao X, Mao R, Liu X, Zhang YM, Li G, Stackman RW, Dong L, Zhang G (2016) Blockade of serotonin 5-HT2A receptors suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in morphine-treated mice. Front Pharmacol 7:514. https://doi.org/10.3389/fphar.2016.00514
Sun J, Tian L, Cui R, Li X (2017) Huperzine A inhibits immediate addictive behavior but not behavioral sensitization following repeated morphine administration in rats. Exp Ther Med. 13:1584–1591. https://doi.org/10.3892/etm.2017.4097
Zarrindast MR, Asgari-Afshar A, Sahebgharani M (2007) Morphine-induced antinociception in the formalin test: Sensitization and interactions with D1 and D2 dopamine receptors and nitric oxide agents. Behav Pharmacol 18:177–184. https://doi.org/10.1097/FBP.0b013e32813c5462
G. Paxinos, C. Watson, (1982) The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition (Google eBook), Elsevier
Pacchioni AM, Gioino G, Assis A, Cancela LM (2002) A single exposure to restraint stress induces behavioral and neurochemical sensitization to stimulating effects of amphetamine: Involvement of NMDA receptors. Ann NY Acad Sci 965:233–246. https://doi.org/10.1111/j.1749-6632.2002.tb04165.x
Del Rosario CN, Pacchioni AM, Cancela LM (2002) Influence of acute or repeated restraint stress on morphine-induced locomotion: involvement of dopamine, opioid and glutamate receptors. Behav Brain Res 134:229–238. https://doi.org/10.1016/S0166-4328(02)00038-4
P.E. Carneiro De Oliveira, R.M. Leão, P.C. Bianchi, M.T. Marin, C. Da Silva Planeta, F.C. Cruz (2016) Stress-induced locomotor sensitization to amphetamine in adult, but not in adolescent rats, is associated with increased expression of ΔFosB in the nucleus accumbens. Front Behav Neurosci 10: 173. https://doi.org/https://doi.org/10.3389/fnbeh.2016.00173.
Rezayof A, Assadpour S, Alijanpour S (2013) Morphine-induced anxiolytic-like effect in morphine-sensitized mice: Involvement of ventral hippocampal nicotinic acetylcholine receptors. Pharmacol Biochem Behav 103:460–466. https://doi.org/10.1016/j.pbb.2012.10.003
Assar N, Mahmoudi D, Mousavi Z, Zarrabian S, Haghparast A (2019) Role of orexin-1 and -2 receptors within the nucleus accumbens in the acquisition of sensitization to morphine in rats. Behav Brain Res 373:112090. https://doi.org/10.1016/j.bbr.2019.112090
Molaei M, Sanati MH, Zaringhalam J, Haghparast A (2014) Microinjection of WIN55,212–2 as a cannabinoid agonist into the basolateral amygdala induces sensitization to morphine in rats. Basic Clin Neurosci 5:295–302
King T, Ossipov MH, Vanderah TW, Porreca F, Lai J (2005) Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? NeuroSignals 14:194–205. https://doi.org/10.1159/000087658
Colvin LA, Bull F, Hales TG (2019) Perioperative opioid analgesia—when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet 393:1558–1568. https://doi.org/10.1016/S0140-6736(19)30430-1
Rowbotham MC, Wallace M (2020) Evolution of analgesic tolerance and opioid-induced hyperalgesia over 6 months: double-blind randomized trial incorporating experimental pain models. J Pain. https://doi.org/10.1016/j.jpain.2020.01.005
Roeckel LA, Utard V, Reiss D, Mouheiche J, Maurin H, Robé A, Audouard E, Wood JN, Goumon Y, Simonin F, Gaveriaux-Ruff C (2017) Morphine-induced hyperalgesia involves mu opioid receptors and the metabolite morphine-3-glucuronide. Sci. Rep. 7:1–15. https://doi.org/10.1038/s41598-017-11120-4
Ahmadi S, Golbaghi H, Azizbeigi R, Esmailzadeh N (2014) N-methyl-D-aspartate receptors involved in morphine-induced hyperalgesia in sensitized mice. Eur J Pharmacol 737:85–90. https://doi.org/10.1016/j.ejphar.2014.04.048
Calcagnetti DJ, Holtzman SG (1990) Factors affecting restraint stress-induced potentiation of morphine analgesia. Brain Res 537:157–162. https://doi.org/10.1016/0006-8993(90)90352-C
Calcagnetti DJ, Holtzman SG (1992) Potentiation of morphine analgesia in rats given a single exposure to restraint stress immobilization. Pharmacol Biochem Behav 41:449–453. https://doi.org/10.1016/0091-3057(92)90125-Y
Calcagnetti DJ, Fleetwood SW, Holtzman SG (1990) Pharmacological profile of the potentiation of opioid analgesia by restraint stress. Pharmacol Biochem Behav 37:193–199. https://doi.org/10.1016/0091-3057(90)90061-L
Isovich E, Mijnster MJ, Flügge G, Fuchs E (2000) Chronic psychosocial stress reduces the density of dopamine transporters. Eur. J Neurosci 12:1071–1078. https://doi.org/10.1046/j.1460-9568.2000.00969.x
Dronjak S, Gavrilovic L (2006) Effects of stress on catecholamine stores in central and peripheral tissues of long-term socially isolated rats. Brazilian J Med Biol Res 39:785–790. https://doi.org/10.1590/S0100-879X2006000600011
Natarajan R, Forrester L, Chiaia NL, Yamamoto BK (2017) Chronic-stress-induced behavioral changes associated with subregion-selective serotonin cell death in the dorsal raphe. J Neurosci 37:6214–6223. https://doi.org/10.1523/JNEUROSCI.3781-16.2017
Hannibal KE, Bishop MD (2014) Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther 94:1816–1825. https://doi.org/10.2522/ptj.20130597
G. Dantas, I.L. Da Silva Torres, L.M. Crema, D.R. Lara, C. Dalmaz (2005) Repeated restraint stress reduces opioid receptor binding in different rat CNS structures. Neurochem Res 30:1–7. https://doi.org/https://doi.org/10.1007/s11064-004-9679-2.
Garcia-Keller C, Martinez SA, Esparza MA, Bollati F, Kalivas PW, Cancela LM (2013) Cross-sensitization between cocaine and acute restraint stress is associated with sensitized dopamine but not glutamate release in the nucleus accumbens. Eur J Neurosci 37:982–995. https://doi.org/10.1111/ejn.12121
Rougé-Pont F, Deroche V, Le Moal M, Piazza PV (1998) Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci 10:3903–3907. https://doi.org/10.1046/j.1460-9568.1998.00438.x
Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, Mcreynolds JR, Robble MA, Vranjkovic O, Wheeler DS, Mantsch JR, Gasser PJ (2013) Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci 33:11800–11810. https://doi.org/10.1523/JNEUROSCI.1969-13.2013
Douma EH, de Kloet ER (2020) Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci Biobehav Rev 108:48–77. https://doi.org/10.1016/j.neubiorev.2019.10.015
Fu Z, Yang H, Xiao Y, Zhao G, Huang H (2012) The gamma-aminobutyric acid type B (GABA B) receptor agonist baclofen inhibits morphine sensitization by decreasing the dopamine level in rat nucleus accumbens. Behav Brain Funct 8:20. https://doi.org/10.1186/1744-9081-8-20
Yamauchi N, Shibasaki T, Wakabayashi I, Demura H (1997) Brain β-endorphin and other opioids are involved in restraint stress- induced stimulation of the hypothalamic-pituitary-adrenal axis, the sympathetic nervous system, and the adrenal medulla in the rat. Brain Res 777:140–146. https://doi.org/10.1016/S0006-8993(97)01097-4
Contet C, Gavériaux-Ruff C, Matifas A, Caradec C, Champy MF, Kieffer BL (2006) Dissociation of analgesic and hormonal responses to forced swim stress using opioid receptor knockout mice. Neuropsychopharmacology 31:1733–1744. https://doi.org/10.1038/sj.npp.1300934
Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, Piazza PV (1995) Stress-induced sensitization and glucocorticoids. I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci 15:7181–7188. https://doi.org/10.1523/jneurosci.15-11-07181.1995
Valenti O, Gill KM, Grace AA (2012) Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: Response alteration by stress pre-exposure. Eur J Neurosci 35:1312–1321. https://doi.org/10.1111/j.1460-9568.2012.08038.x
Lataster J, Collip D, Ceccarini J, Haas D, Booij L, van Os J, Pruessner J, Van Laere K, Myin-Germeys I (2011) Psychosocial stress is associated with in vivo dopamine release in human ventromedial prefrontal cortex: a positron emission tomography study using [18F] fallypride. Neuroimage 58:1081–1089
Stöhr T, Almeida OFX, Landgraf R, Shippenberg TS, Holsboer F, Spanagel R (1999) Stress- and corticosteroid-induced modulation of the locomotor response to morphine in rats. Behav Brain Res 103:85–93. https://doi.org/10.1016/S0166-4328(99)00027-3
Costa A, Smeraldi A, Tassorelli C, Greco R, Nappi G (2005) Effects of acute and chronic restraint stress on nitroglycerin-induced hyperalgesia in rats. Neurosci Lett 383:7–11
Borgkvist A, Valjent E, Santini E, Hervé D, Girault JA, Fisone G (2008) Delayed, context- and dopamine D1 receptor-dependent activation of ERK in morphine-sensitized mice. Neuropharmacology 55:230–237. https://doi.org/10.1016/j.neuropharm.2008.05.028
Stout KA, Dunn AR, Lohr KM, Alter SP, Cliburn RA, Guillot TS, Miller GW (2016) Selective Enhancement of Dopamine Release in the Ventral Pallidum of Methamphetamine-Sensitized Mice. ACS Chem Neurosci 7:1364–1373. https://doi.org/10.1021/acschemneuro.6b00131
Listos J, Łupina M, Talarek S, Mazur A, Orzelska-Górka J, Kotlińska J (2019) The mechanisms involved in morphine addiction: an overview. Int J Mol Sci 20:4302. https://doi.org/10.3390/ijms20174302
Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ (2004) Induction of ΔFosB in reward-related brain structures after chronic stress. J Neurosci 24:10594–10602. https://doi.org/10.1523/JNEUROSCI.2542-04.2004
Vanderschuren LJMJ, Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 151:99–120. https://doi.org/10.1007/s002130000493
Kelz MB, Chen J, Carlezon WA, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeler DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ (1999) Expression of the transcription factor ΔFosB in the brain controls sensitivity to cocaine. Nature 401:272–276. https://doi.org/10.1038/45790
Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM (2013) Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator. J Neurosci 33:3434–3442. https://doi.org/10.1523/JNEUROSCI.4881-12.2013
Lohman RJ, Liu L, Morris M, O’Brien TJ (2005) Validation of a method for localised microinjection of drugs into thalamic subregions in rats for epilepsy pharmacological studies. J Neurosci Methods 146:191–197. https://doi.org/10.1016/j.jneumeth.2005.02.008
Acknowledgements
The Vice-Chancellor for Research & Technology of Shahid Beheshti University of Medical Sciences supported this work (Grant No. 98-21484-1398/12/20). Also, the authors would like to appreciate the Neuroscience Research Center, School of Medicine, Shahid Beheshti University of Medical Sciences, for their cooperation in carrying out this study.
Author information
Authors and Affiliations
Contributions
AH: Conceptualization, Methodology, Writing-Reviewing, and Editing. EC: Data curation, Data analysis, Writing-Original draft preparation. GF: Writing-Original draft preparation. MR: Writing-Original draft preparation, Writing-Reviewing, and Editing. MZ: Supervision. All authors critically reviewed content and approved the final version for publication.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Charmchi, E., Faramarzi, G., Rashvand, M. et al. Restraint Stress Potentiated Morphine Sensitization: Involvement of Dopamine Receptors within the Nucleus Accumbens. Neurochem Res 46, 648–659 (2021). https://doi.org/10.1007/s11064-020-03199-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03199-5