Abstract
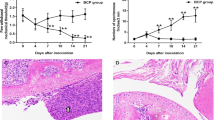
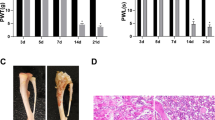
Mas-related G-protein-coupled receptor subtype C (MrgC) has been shown to play an important role in the development of bone cancer pain. Ubiquitination is reported to participate in pain. However, whether MrgC ubiquitination plays a role in bone cancer pain remains unclear. To answer this question, we designed and performed this study. Osteosarcoma cells were implanted into the intramedullary space of the right femurs of C3H/HeJ mice to induce progressive bone cancer pain. MrgC agonist bovine adrenal medulla 8–22 (BAM 8–22) or MrgC antagonist anti-MrgC antibody were injected intrathecally on day 14 after bone cancer pain was successfully induced. The pain behaviors, the MrgC ubiquitination levels and intracellular calcium concentration in spinal neurons were measured before and after injection, respectively. With comparison to normal and sham group, mice in tumor group exhibited serious bone cancer pain on day 14, and the level of MrgC ubiquitination and intracellular calcium concentration in spinal neurons was significantly higher. Intrathecal injection of BAM 8–22 significantly alleviated bone cancer pain, increased the MrgC ubiquitination level and decreased intracellular calcium concentration in spinal neurons; however, these effects were reversed by administration of anti-MrgC antibody. Our study reveals that MrgC ubiquitination participates in the production and maintenance of bone cancer pain in mice, possibly through the regulation of intracellular calcium concentration in mice spinal neurons.




Similar content being viewed by others
References
Mantyh P (2013) Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain 154:S54–S62. https://doi.org/10.1016/j.pain.2013.07.044
Oladosu FA, Maixner W, Nackley AG (2015) Alternative splicing of G protein-coupled receptors: relevance to pain management. Mayo Clin Proc 90(8):1135–1151. https://doi.org/10.1016/j.mayocp.2015.06.010
Geppetti P, Veldhuis NA, Lieu T, Bunnett NW (2015) G protein-coupled receptors: dynamic machines for signaling pain and itch. Neuron 88(4):635–649. https://doi.org/10.1016/j.neuron.2015.11.001
Wang D, Chen T, Zhou X, Couture R, Hong Y (2013) Activation of Mas oncogene-related gene (Mrg) C receptors enhances morphine-induced analgesia through modulation of coupling of mu-opioid receptor to Gi-protein in rat spinal dorsal horn. Neuroscience 253:455–464. https://doi.org/10.1016/j.neuroscience.2013.08.069
Zylka MJ, Dong X, Southwell AL, Anderson DJ (2003) Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci USA 100(17):10043–10048. https://doi.org/10.1073/pnas.1732949100
Li Z, He SQ, Xu Q, Yang F, Tiwari V, Liu Q, Tang Z, Han L, Chu YX, Wang Y, Hin N, Tsukamoto T, Slusher B, Guan X, Wei F, Raja SN, Dong X, Guan Y (2014) Activation of MrgC receptor inhibits N-type calcium channels in small-diameter primary sensory neurons in mice. Pain 155(8):1613–1621. https://doi.org/10.1016/j.pain.2014.05.008
Lembo PM, Grazzini E, Groblewski T, O’Donnell D, Roy MO, Zhang J, Hoffert C, Cao J, Schmidt R, Pelletier M, Labarre M, Gosselin M, Fortin Y, Banville D, Shen SH, Strom P, Payza K, Dray A, Walker P, Ahmad S (2002) Proenkephalin A gene products activate a new family of sensory neuron–specific GPCRs. Nat Neurosci 5(3):201–209. https://doi.org/10.1038/nn815
He SQ, Xu Q, Tiwari V, Yang F, Anderson M, Chen Z, Grenald SA, Raja SN, Dong X, Guan Y (2018) Oligomerization of MrgC11 and mu-opioid receptors in sensory neurons enhances morphine analgesia. Sci Signal 11(535):eaao3134. https://doi.org/10.1126/scisignal.aao3134
Tiwari V, Yang F, He SQ, Shechter R, Zhang C, Shu B, Zhang T, Tiwari V, Wang Y, Dong X, Guan Y, Raja SN (2016) Activation of peripheral mu-opioid receptors by dermorphin [D-Arg2, Lys4] (1-4) amide leads to modality-preferred inhibition of neuropathic pain. Anesthesiology 124(3):706–720. https://doi.org/10.1097/ALN.0000000000000993
Wang D, Wang P, Jiang J, Lv Q, Zeng X, Hong Y (2015) Activation of Mas oncogene-related G protein-coupled receptors inhibits neurochemical alterations in the spinal dorsal horn and dorsal root ganglia associated with inflammatory pain in rats. J Pharmacol Exp Ther 354(3):431–439. https://doi.org/10.1124/jpet.115.225672
He SQ, Han L, Li Z, Xu Q, Tiwari V, Yang F, Guan X, Wang Y, Raja SN, Dong X, Guan Y (2014) Temporal changes in MrgC expression after spinal nerve injury. Neuroscience 261:43–51. https://doi.org/10.1016/j.neuroscience.2013.12.041
He SQ, Li Z, Chu YX, Han L, Xu Q, Li M, Yang F, Liu Q, Tang Z, Wang Y, Hin N, Tsukamoto T, Slusher B, Tiwari V, Shechter R, Wei F, Raja SN, Dong X, Guan Y (2014) MrgC agonism at central terminals of primary sensory neurons inhibits neuropathic pain. Pain 155(3):534–544. https://doi.org/10.1016/j.pain.2013.12.008
Sun Y, Zhang J, Lei Y, Lu C, Hou B, Ma Z, Gu X (2016) Activation of spinal MrgC-Gi-NR2B-nNOS signaling pathway by Mas oncogene-related gene C receptor agonist bovine adrenal medulla 8–22 attenuates bone cancer pain in mice. Am J Transl Res 8(2):1144–1154
Sun YE, Lu CE, Lei Y, Liu Y, Ma Z, Gu X (2015) Mas-related G-protein-coupled receptor c agonist bovine adrenal medulla 8–22 attenuates bone cancer pain in mice. Int J Clin Exp Med 8(11):20178–20187
Li Z, He SQ, Tseng PY, Xu Q, Tiwari V, Yang F, Shu B, Zhang T, Tang Z, Raja SN, Wang Y, Dong X, Guan Y (2015) The inhibition of high-voltage-activated calcium current by activation of MrgC11 involves phospholipase C-dependent mechanisms. Neuroscience 300:393–403. https://doi.org/10.1016/j.neuroscience.2015.05.043
Kostenis E, Martini L, Ellis J, Waldhoer M, Heydorn A, Rosenkilde MM, Norregaard PK, Jorgensen R, Whistler JL, Milligan G (2005) A highly conserved glycine within linker I and the extreme C terminus of G protein alpha subunits interact cooperatively in switching G protein-coupled receptor-to-effector specificity. J Pharmacol Exp Ther 313(1):78–87. https://doi.org/10.1124/jpet.104.080424
Foot N, Henshall T, Kumar S (2017) Ubiquitination and the regulation of membrane proteins. Physiol Rev 97(1):253–281. https://doi.org/10.1152/physrev.00012.2016
Kalayil S, Bhogaraju S, Bonn F, Shin D, Liu Y, Gan N, Basquin J, Grumati P, Luo ZQ, Dikic I (2018) Insights into catalysis and function of phosphoribosyl-linked serine ubiquitination. Nature 557(7707):734–738. https://doi.org/10.1038/s41586-018-0145-8
Kong F, Liu Z, Jain VG, Shima K, Suzuki T, Muglia LJ, Starczynowski DT, Pasare C, Bhattacharyya S (2017) Inhibition of IRAK1 ubiquitination determines glucocorticoid sensitivity for TLR9-induced inflammation in macrophages. J Immunol 199(10):3654–3667. https://doi.org/10.4049/jimmunol.1700443
Popovic D, Vucic D, Dikic I (2014) Ubiquitination in disease pathogenesis and treatment. Nat Med 20(11):1242–1253. https://doi.org/10.1038/nm.3739
Lai CY, Ho YC, Hsieh MC, Wang HH, Cheng JK, Chau YP, Peng HY (2016) Spinal Fbxo3-dependent Fbxl2 ubiquitination of active zone protein RIM1alpha mediates neuropathic allodynia through CaV2.2 activation. J Clin Neurosci NLM 36(37):9722–9738. https://doi.org/10.1523/jneurosci.1732-16.2016
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16(2):109–110
Schwei Matthew J, Honore Prisca, Ramnaraine Margaret L, Rogers Scott D, Clohisy Denis R, Salak-Johnson Janeen L, Mantyh PW (1999) Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci 19(24):10886–10897
Jiang J, Wang D, Zhou X, Huo Y, Chen T, Hu F, Quirion R, Hong Y (2013) Effect of Mas-related gene (Mrg) receptors on hyperalgesia in rats with CFA-induced inflammation via direct and indirect mechanisms. Br J Pharmacol 170(5):1027–1040. https://doi.org/10.1111/bph.12326
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative Assessment of Tactile Allodynia in the Rat Paw. J Neurosci Meth 53(1):55–63. https://doi.org/10.1016/0165-0270(94)90144-9
Glare PA, Davies PS, Finlay E, Gulati A, Lemanne D, Moryl N, Oeffinger KC, Paice JA, Stubblefield MD, Syrjala KL (2014) Pain in cancer survivors. J Clin Oncol 32(16):1739–1747. https://doi.org/10.1200/JCO.2013.52.4629
Gu X, Zhang J, Ma Z, Wang J, Zhou X, Jin Y, Xia X, Gao Q, Mei F (2010) The role of N-methyl-D-aspartate receptor subunit NR2B in spinal cord in cancer pain. Eur J Pain 14(5):496–502. https://doi.org/10.1016/j.ejpain.2009.09.001
Ren BX, Gu XP, Zheng YG, Liu CL, Wang D, Sun YE, Ma ZL (2012) Intrathecal injection of metabotropic glutamate receptor subtype 3 and 5 agonist/antagonist attenuates bone cancer pain by inhibition of spinal astrocyte activation in a mouse model. Anesthesiology 116(1):122–132. https://doi.org/10.1097/ALN.0b013e31823de68d
Raehal KM, Schmid CL, Groer CE, Bohn LM (2011) Functional selectivity at the mu-opioid receptor: implications for understanding opioid analgesia and tolerance. Pharmacol Rev 63(4):1001–1019. https://doi.org/10.1124/pr.111.004598
Liao Y, Xu M (2017) Efficacy and mechanism of action of etanercept in bone cancer pain. Pharmazie 72(4):219–222. https://doi.org/10.1691/ph.2017.6901
Lee JA, Xing Y, Nguyen D, Xie J, Lee CJ, Black DL (2007) Depolarization and CaM kinase IV modulate NMDA receptor splicing through two essential RNA elements. PLoS Biol 5(2):e40. https://doi.org/10.1371/journal.pbio.0050040
O’Leary H, Liu WH, Rorabaugh JM, Coultrap SJ, Bayer KU (2011) Nucleotides and phosphorylation bi-directionally modulate Ca2 +/calmodulin-dependent protein kinase II (CaMKII) binding to the N-methyl-D-aspartate (NMDA) receptor subunit GluN2B. J Biol Chem 286(36):31272–31281. https://doi.org/10.1074/jbc.M111.233668
Wild AR, Jones S, Gibb AJ (2014) Activity-dependent regulation of NMDA receptors in substantia nigra dopaminergic neurones. J Physiol 592(4):653–668. https://doi.org/10.1113/jphysiol.2013.267310
Liu Y, Cui X, Sun YE, Yang X, Ni K, Zhou Y, Ma Z, Gu X (2014) Intrathecal injection of the peptide myr-NR2B9c attenuates bone cancer pain via perturbing N-methyl-D-aspartate receptor-PSD-95 protein interactions in mice. Anesth Analg 118(6):1345–1354. https://doi.org/10.1213/ANE.0000000000000202
Pickart CM, Eddins MJ (2004) Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta 1695(1–3):55–72. https://doi.org/10.1016/j.bbamcr.2004.09.019
Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82(2):373–428. https://doi.org/10.1152/physrev.00027.2001
Kenakin T (2009) Biased agonism. F1000 Biol Rep 1:87. https://doi.org/10.3410/b1-87
Critchley WR, Pellet-Many C, Ringham-Terry B, Harrison MA, Zachary IC, Ponnambalam S (2018) Receptor tyrosine kinase ubiquitination and de-ubiquitination in signal transduction and receptor trafficking. Cells 7(3):22. https://doi.org/10.3390/cells7030022
Hsieh MC, Ho YC, Lai CY, Chou D, Chen GD, Lin TB, Peng HY (2018) Spinal TNF-alpha impedes Fbxo45-dependent Munc13-1 ubiquitination to mediate neuropathic allodynia in rats. Cell Death Dis 9(8):811. https://doi.org/10.1038/s41419-018-0859-4
Cachemaille M, Laedermann CJ, Pertin M, Abriel H, Gosselin RD, Decosterd I (2012) Neuronal expression of the ubiquitin ligase Nedd4-2 in rat dorsal root ganglia: modulation in the spared nerve injury model of neuropathic pain. Neuroscience 227:370–380. https://doi.org/10.1016/j.neuroscience.2012.09.044
Cheng W, Chen YL, Wu L, Miao B, Yin Q, Wang JF, Fu ZJ (2016) Inhibition of spinal UCHL1 attenuates pain facilitation in a cancer-induced bone pain model by inhibiting ubiquitin and glial activation. Am J Transl Res 8(7):3041–3048
Funding
This work was supported by National Natural Science Foundation of China (Nos. 81870871, 81400914) and Key Project supported by Medical Science and Technology Development Foundation, Nanjing Department of Health (QRX17138, ZKX18018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
This experiment was approved by the Institution of Animal Care and Usage Committee at the Medical School of Nanjing University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, YE., Xu, HY., Hao, J. et al. The Ubiquitination of Spinal MrgC Alleviates Bone Cancer Pain and Reduces Intracellular Calcium Concentration in Spinal Neurons in Mice. Neurochem Res 44, 2527–2535 (2019). https://doi.org/10.1007/s11064-019-02869-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02869-3