Abstract
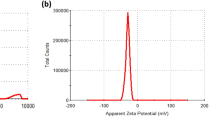
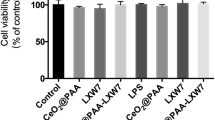
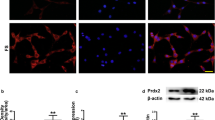
CeO2 nanoparticles (nanoceria) have been used in many studies as a powerful free radical scavenger, and LXW7, a small-molecule peptide, can specifically target the integrin αvβ3, whose neuroprotective effects have also been demonstrated. The objective of this study is to observe the neuroprotective effect and potential mechanism of CeO2@PAA-LXW7, a new compound that couples CeO2@PAA (nanoceria modified with the functional group of polyacrylic acid) with LXW7 via a series of chemical reactions, in H2O2-induced NGF-differentiated PC12 cells. We examined the effects of LXW7, CeO2@PAA, and CeO2@PAA-LXW7 on the viability of primary hippocampal neurons and found that there was no significant difference under control conditions, but increased cellular viability was observed in the case of H2O2-induced injury. We used H2O2-induced NGF-differentiated PC12 cells as the classical injury model to investigate the neuroprotective effect of CeO2@PAA-LXW7. In this study, LXW7, CeO2@PAA, and CeO2@PAA-LXW7 inhibit H2O2-induced oxidative stress by reducing the production of reactive oxygen species (ROS) and regulating Bax/Bcl-2, cleaved caspase-3 and mitochondrial cytochrome C (cyto C) in the apoptotic signaling pathways. We found that the levels of phosphorylation of focal adhesion kinase (FAK) and of signal transducer and activator of transcription 3 (STAT3) increased significantly in H2O2-induced NGF-differentiated PC12 cells, whereas LXW7, CeO2@PAA, and CeO2@PAA-LXW7 suppressed the increase to different degrees. Among the abovementioned changes, the inhibitory effect of CeO2@PAA-LXW7 on H2O2-induced changes, including the increases in the levels of p-FAK and p-STAT3, is more obvious than that of LXW7 or CeO2@PAA alone. In summary, these results suggest that integrin signaling participates in the regulation of apoptosis via the regulation of ROS and of the apoptosis pathway in H2O2-induced NGF-differentiated PC12 cells. LXW7, CeO2@PAA, and CeO2@PAA-LXW7 can play neuroprotective roles by counteracting the oxidative stress and apoptosis induced by H2O2 in NGF-differentiated PC12 cells. CeO2@PAA-LXW7 exerting a more powerful synergistic effect via the conjunction of LXW7 and CeO2@PAA.










Similar content being viewed by others
References
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18(9):685–716
Armogida M, Nisticò R, Mercuri NB (2012) Therapeutic potential of targeting hydrogen peroxide metabolism in the treatment of brain ischaemia. Br J Pharmacol 166(4):1211–1224
Cornelius C et al (2013) Traumatic brain injury: oxidative stress and neuroprotection. Antioxid Redox Signal 19(8):836–853
Melo A et al (2011) Oxidative stress in neurodegenerative diseases: mechanisms and therapeutic perspectives. Oxid Med Cell Longev. https://doi.org/10.1155/2011/467180
Paloczi J et al (2017) Neuroprotection in oxidative stress-related neurodegenerative diseases: role of endocannabinoid system modulation. Antioxid Redox Signal. https://doi.org/10.1089/ars.2017.7144
Luo P et al (2012) Protective effect of Homer 1a against hydrogen peroxide-induced oxidative stress in PC12 cells. Free Radic Res 46(6):766–776
Yan SH et al (2018) Modulations of Keap1-Nrf2 signaling axis by TIIA ameliorated the oxidative stress-induced myocardial apoptosis. Free Radical Biol Med 115:191–201
Li P et al (2018) Serum exosomes attenuate H2O2-induced apoptosis in Rat H9C2 cardiomyocytes via ERK1/2. J Cardiovasc Transl Res. https://doi.org/10.1007/s12265-018-9791-3
Huang WY et al (2018) Protective effects of blueberry anthocyanins against H2O2-induced oxidative injuries in human retinal pigment epithelial cells. J Agric Food Chem 66:1638–1648
Hecquet CM et al (2008) Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res 102(3):347–355
Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110(6):673
Mazalouskas M et al (2015) Integrin β3 haploinsufficiency modulates serotonin transport and antidepressant-sensitive behavior in mice. Neuropsychopharmacology 40(8):2015
Wu H et al (2013) Imaging integrin α(v)β(3) positive glioma with a novel RGD dimer probe and the impact of antiangiogenic agent (Endostar) on its tumor uptake. Cancer Lett 335(1):75–80
Chen WT et al (2012) Integrin αvβ3-targeted dynamic contrast-enhanced magnetic resonance imaging using a gadolinium-loaded polyethylene gycol-dendrimer-cyclic RGD conjugate to evaluate tumor angiogenesis and to assess early antiangiogenic treatment response in a mouse xenogr. Mol Imaging 11(4):286
Diao Y et al (2012) Designed synthetic analogs of the α-helical peptide temporin-La with improved antitumor efficacies via charge modification and incorporation of the integrin αvβ3 homing domain. J Pept Sci 18(7):476–486
Gao Y et al (2015) Enhanced antitumor efficacy by cyclic RGDyK-conjugated and paclitaxel-loaded pH-responsive polymeric micelles. Acta Biomater 23:127–135
Yasuda J, Okada M, Yamawaki H (2017) T3 peptide, an active fragment of tumstatin, inhibits H2O2-induced apoptosis in H9c2 cardiomyoblasts. Eur J Pharmacol 807:64–70
Liu J et al (2016) High glucose regulates LN expression in human liver sinusoidal endothelial cells through ROS/integrin αvβ3 pathway. Environ Toxicol Pharmacol 42:231
Visavadiya NP et al (2016) Integrin-FAK signaling rapidly and potently promotes mitochondrial function through STAT3. Cell Commun Signal 14(1):32
Xu CS et al (2015) Involvement of ROS-alpha v beta 3 integrin-FAK/Pyk2 in the inhibitory effect of melatonin on U251 glioma cell migration and invasion under hypoxia. J Transl Med 13(1):1–11
Hao D et al (2017) Discovery and characterization of a potent and specific peptide ligand targeting endothelial progenitor cells and endothelial cells for tissue regeneration. ACS Chem Biol 12(4):1075–1086
Xiao W et al (2010) The use of one-bead one-compound combinatorial library technology to discover high-affinity alphavbeta3 integrin and cancer targeting arginine-glycine-aspartic acid ligands with a built-in handle. Mol Cancer Ther 9(10):2714–2723
Westerink RH, Ewing AG (2008) The PC12 cell as model for neurosecretion. Acta Physiol 192(2):273–285
Tischler AS et al (1983) Glucocorticoids increase catecholamine synthesis and storage in PC 12 pheochromocytoma cell cultures. J Neurochem 40(2):364 – 70
Beaudoin GM III et al (2012) Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat Protoc 7(9):1741–1754
Kuszczyk MA et al (2014) 1MeTIQ provides protection against Aβ-induced reduction of surface expression of synaptic proteins and inhibits H2O2-induced oxidative stress in primary hippocampal neurons. Neurotox Res 25(4):348
Boufker HI et al (2010) The Src inhibitor dasatinib accelerates the differentiation of human bone marrow-derived mesenchymal stromal cells into osteoblasts. BMC Cancer 10(1):298
Liu B et al (2012) Investigation of catalytic mechanism of formaldehyde oxidation over three-dimensionally ordered macroporous Au/CeO2 catalyst. Appl Catal B Environ 111(2):467–475
Liu B et al (2012) Three-dimensionally ordered macroporous Au/CeO2–Co3O4 catalysts with nanoporous walls for enhanced catalytic oxidation of formaldehyde. Appl Catal B Environ 127:47–58
Sreeremya TS et al (2012) A novel aqueous route to fabricate ultrasmall monodisperse lipophilic cerium oxide nanoparticles. Ind Eng Chem Res 51(1):318–326
Guo Y, Wu P (2008) FTIR spectroscopic study of the acrylamide states in AOT reversed micelles. J Mol Struct 883:31–37
Alekseev SA et al (2007) Fourier transform infrared spectroscopy and temperature-programmed desorption mass spectrometry study of surface chemistry of porous 6H–SiC. Chem Mater 19(9):2189–2194
Huang M, Khor E, Lim LY (2004) Uptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylation. Pharm Res 21(2):344–353
Yamazoe S et al (2007) Mechanism of photo-oxidation of NH3 over TiO2: Fourier transform infrared study of the intermediate species. J Phys Chem C 111(29):392–395
Hosseini A et al (2015) Cerium and yttrium oxide nanoparticles against lead-induced oxidative stress and apoptosis in rat hippocampus. Biol Trace Elem Res 164(1):80–89
Estevez AY et al (2011) Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic Biol Med 51(6):1155–1163
Fang T et al (2016) LXW7 ameliorates focal cerebral ischemia injury and attenuates inflammatory responses in activated microglia in rats. Braz J Med Biol Res 49(9):e5287
Wang Y et al (2016) Optimization of RGD-containing cyclic peptides against alphavbeta3 Integrin. Mol Cancer Ther 15(2):232–240
Greene LA, Tischler AS (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 73(7):2424–2428
Olanow CW (1993) A radical hypothesis for neurodegeneration. Trends Neurosci 16(11):439–444
Behl C (1999) Alzheimer’s disease and oxidative stress: implications for novel therapeutic approaches. Prog Neurobiol 57(3):301–323
Liu Z et al. (2017) Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid Med Cell Longev. https://doi.org/10.1155/2017/2525967
Satoh T et al (1996) Free radical-independent protection by nerve growth factor and Bcl-2 of PC12 cells from hydrogen peroxide-triggered apoptosis. J Biochem 120(3):540–546
Bartosz G (2000) Free radicals in biology and medicine. Cell Biol Int 24(10):764
Franco R, Cidlowski JA (2009) Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ 16(10):1303–1314
Piro S et al (2002) Chronic exposure to free fatty acids or high glucose induces apoptosis in rat pancreatic islets: Possible role of oxidative stress. Metabolism 51(10):1340–1347
Hasnan J et al (2010) Relationship between apoptotic markers (Bax and Bcl-2) and biochemical markers in type 2 diabetes mellitus. Singap Med J 51(1):50–55
Hampton MB, Fadeel B, Orrenius S (1998) Redox regulation of the caspases during apoptosis. Ann N Y Acad Sci 854(1):328–335
Allen DA, Yaqoob MM, Harwood SM (2005) Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J Nutr Biochem 16(12):705–713
Simon HU, Hajyehia A, Levischaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5(5):415
Ricci JE, Gottlieb RA, Green DR (2003) Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J Cell Biol 160(1):65
Kuribayashi K, Mayes PA, Eldeiry WS (2006) What are caspases 3 and 7 doing upstream of the mitochondria? Cancer Biol Ther 5(7):763
Pourkhalili N et al (2011) Biochemical and cellular evidence of the benefit of a combination of cerium oxide nanoparticles and selenium to diabetic rats. World J Diabetes 2(11):204–210
Pirmohamed T et al (2010) Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun (Camb) 46(16):2736–2738
Clark A et al (2011) Cerium oxide and platinum nanoparticles protect cells from oxidant-mediated apoptosis. J Nanopart Res 13(10):5547–5555
Celardo I et al (2011) Ce3+ ions determine redox-dependent anti-apoptotic effect of cerium oxide nanoparticles. ACS Nano 5(6):4537–4549
Li C et al (2014) Cytotoxicity of ultrafine monodispersed nanoceria on human gastric cancer cells. J Biomed Nanotechnol 10(7):1231
Zhang T et al (2018) Combination therapy with LXW7 and ceria nanoparticles protects against acute cerebral ischemia/reperfusion injury in rats. Curr Med Sci 38(1):144–152
Meredith JE, Schwartz MA (1997) Integrins, adhesion and apoptosis. Trends Cell Biol 7(4):146
Stupack DG, Cheresh DA (2002) Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci 115(Pt 19):3729
Aguzzi MS et al (2004) RGDS peptide induces caspase 8 and caspase 9 activation in human endothelial cells. BMC Pregnancy Childbirth 103(11):4180
Serpente N, Birling MC, Price J (1996) The regulation of the expression, phosphorylation, and protein associations of pp125FAK during rat brain development. Mol Cell Neurosci 7(5):391–403
Shimamura N et al (2006) Inhibition of integrin alphavbeta3 ameliorates focal cerebral ischemic damage in the rat middle cerebral artery occlusion model. Stroke 37(7):1902–1909
Parsons JT (2003) Focal adhesion kinase: the first ten years. J Cell Sci 116(8):1409–1416
Tornatore TF et al (2011) A role for focal adhesion kinase in cardiac mitochondrial biogenesis induced by mechanical stress. Am J Physiol Heart Circ Physiol 300(3):H902-12
Keasey MP et al (2013) Inhibition of a novel specific neuroglial integrin signaling pathway increases STAT3-mediated CNTF expression. Cell Commun Signal 11(1):35
Banerjee K et al (2017) Reduced FAK-STAT3 signaling contributes to ER stress-induced mitochondrial dysfunction and death in endothelial cells. Cell Signal 36:154–162
Manimaran M, Rajkumar T (2014) Survival response of hippocampal neurons under low oxygen conditions induced by Hippophae rhamnoidesis associated with JAK/STAT signaling. PLoS ONE 9(2):e87694
Song Y et al (2013) Protective effect of Ginkgolide B against acute spinal cord injury in rats and its correlation with the JAK/STAT signaling pathway. Neurochem Res 38(3):610–619
Garza JC et al (2008) Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem 283(26):18238
Gough DJ et al (2009) Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science 324(5935):1713–1716
Boengler K et al (2010) Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol 105(6):771–785
Wang C, Youle RJ (1999) The role of mitochondria in apoptosis. BMB Rep 35(1):11
Poli G et al (2004) Oxidative stress and cell signalling. Curr Med Chem 11(9):1163
Cheng X et al (2011) Stage-dependent STAT3 activation is involved in the differentiation of rat hippocampus neural stem cells. Neurosci Lett 493(1–2):18–23
Acknowledgements
This study was funded by Health and Family Planning Commission of Shenzhen Municipality Fund (No. 201401027). The drug synthetic process was supported by the Inner Mongolia University Startup Fund Project (21300-5145152), the Inner Mongolia Education Department Key Project (NJZZ16015), and the Inner Mongolia Natural Science Foundation (2016MS0216).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflicts of interest in this work.
Rights and permissions
About this article
Cite this article
Jia, J., Zhang, T., Chi, J. et al. Neuroprotective Effect of CeO2@PAA-LXW7 Against H2O2-Induced Cytotoxicity in NGF-Differentiated PC12 Cells. Neurochem Res 43, 1439–1453 (2018). https://doi.org/10.1007/s11064-018-2559-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2559-y