Abstract
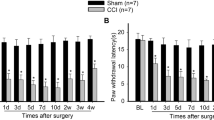
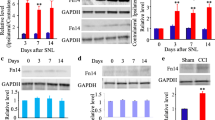
Neuropathic pain is a complicated clinical syndrome caused by heterogeneous etiology. Despite the fact that the underlying mechanisms remain elusive, it is well accepted that neuroinflammation plays a critical role in the development of neuropathic pain. Fascin-1, an actin-bundling protein, has been proved to be involved in the processing of diverse biological events including cellular development, immunity, and tumor invasion etc. Recent studies have shown that Fascin-1 participates in antigen presentation and the regulation of pro-inflammatory agents. However, whether Fascin-1 is involved in neuropathic pain has not been reported. In the present study we examined the potential role of Fascin-1 by using a rodent model of chronic constriction injury (CCI). Our results showed that Fascin-1 increased rapidly in dorsal root ganglions (DRG) and spinal cord (SC) after CCI. The increased Fascin-1 widely expressed in DRG, however, it localized predominantly in microglia, seldom in neuron, and hardly in astrocyte in the SC. Intrathecal injection of Fascin-1 siRNA not only suppressed the activation of microglia and the release of pro-inflammatory mediators, but also attenuated the mechanical allodynia and thermal hyperalgesia induced by CCI.





Similar content being viewed by others
References
Backonja MM (2003) Defining neuropathic pain. Anesth Analg 97:785–790
Zimmermann M (2001) Pathobiology of neuropathic pain. Eur J Pharmacol 429:23–37
Torrance N, Ferguson JA, Afolabi E, et al (2013) Neuropathic pain in the community: more under-treated than refractory? Pain 154:690–699
Rice AS, Hill RG (2006) New treatments for neuropathic pain. Annu Rev Med 57:535–551
Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M (2015) Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 14:162–173
Mika J, Osikowicz M, Rojewska E, Korostynski M, Wawrzczak-Bargiela A, Przewlocki R, Przewlocka B (2009) Differential activation of spinal microglial and astroglial cells in a mouse model of peripheral neuropathic pain. Eur J Pharmacol 623:65–72
Raghavendra V, Tanga F, DeLeo JA (2003) Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther 306:624–630
Nakajima K, Kohsaka S (2001) Microglia: activation and their significance in the central nervous system. J Biochem 130:169–175
Vallejo R, Tilley DM, Vogel L, Benyamin R (2010) The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract Off J World Inst Pain 10:167–184
Beggs S, Salter MW (2007) Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav Immun 21:624–633
Hains BC, Waxman SG (2006) Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci Off J Soc Neurosci 26:4308–4317
Sama MA, Mathis DM, Furman JL, Abdul HM, Artiushin IA, Kraner SD, Norris CM (2008) Interleukin-1beta-dependent signaling between astrocytes and neurons depends critically on astrocytic calcineurin/NFAT activity. J Biol Chem 283:21953–21964
Schubert P, Morino T, Miyazaki H, Ogata T, Nakamura Y, Marchini C, Ferroni S (2000) Cascading glia reactions: a common pathomechanism and its differentiated control by cyclic nucleotide signaling. Ann N Y Acad Sci 903:24–33
Leung L, Cahill CM (2010) TNF-alpha and neuropathic pain: a review. J Neuroinflamm 7:27
Wei XH, Na XD, Liao GJ, Chen QY, Cui Y, Chen FY, Li YY, Zang Y, Liu XG (2013) The up-regulation of IL-6 in DRG and spinal dorsal horn contributes to neuropathic pain following L5 ventral root transection. Exp Neurol 241:159–168
Hashimoto Y, Kim DJ, Adams JC (2011) The roles of fascins in health and disease. J Pathol 224:289–300
Adams JC (2004) Roles of fascin in cell adhesion and motility. Curr Opin Cell Biol 16:590–596
De Arcangelis A, Georges-Labouesse E, Adams JC (2004) Expression of fascin-1, the gene encoding the actin-bundling protein fascin-1, during mouse embryogenesis. Gene Expr Patterns 4:637–643
Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC (2002) Fascins, and their roles in cell structure and function. BioEssays 24:350–361
Pak CW, Flynn KC, Bamburg JR (2008) Actin-binding proteins take the reins in growth cones. Nat Rev Neurosci 9:136–147
Kim JK, Lee SM, Suk K, Lee WH (2011) A novel pathway responsible for lipopolysaccharide-induced translational regulation of TNF-alpha and IL-6 expression involves protein kinase C and fascin. J Immunol 187:6327–6334
Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Capello E, Mancardi GL, Aloisi F (2006) Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake, and interaction with proliferating T cells. J Neuropathol Exp Neurol 65:124–141
Almolda B, Gonzalez B, Castellano B (2010) Activated microglial cells acquire an immature dendritic cell phenotype and may terminate the immune response in an acute model of EAE. J Neuroimmunol 223:39–54
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63
Otto JJ (1994) Actin-bundling proteins. Curr Opin Cell Biol 6:105–109
Al-Alwan MM, Rowden G, Lee TD, West KA (2001) Fascin is involved in the antigen presentation activity of mature dendritic cells. J Immunol 166:338–345
Nagel J, Delandre C, Zhang Y, Forstner F, Moore AW, Tavosanis G (2012) Fascin controls neuronal class-specific dendrite arbor morphology. Development 139:2999–3009
Meller R, Thompson SJ, Lusardi TA, Ordonez AN, Ashley MD, Jessick V, Wang W, Torrey DJ, Henshall DC, Gafken PR, Saugstad JA, Xiong ZG, Simon RP (2008) Ubiquitin proteasome-mediated synaptic reorganization: a novel mechanism underlying rapid ischemic tolerance. J Neurosci Off J Soc Neurosci 28:50–59
Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR (2006) A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci Off J Soc Neurosci 26:3551–3560
Komori N, Takemori N, Kim HK, Singh A, Hwang SH, Foreman RD, Chung K, Chung JM, Matsumoto H (2007) Proteomics study of neuropathic and nonneuropathic dorsal root ganglia: altered protein regulation following segmental spinal nerve ligation injury. Physiol Genom 29:215–230
Yadav R, Weng HR (2017) EZH2 regulates spinal neuroinflammation in rats with neuropathic pain. Neuroscience 349:106–117
Balkowiec-Iskra E, Vermehren-Schmaedick A, Balkowiec A (2011) Tumor necrosis factor-alpha increases brain-derived neurotrophic factor expression in trigeminal ganglion neurons in an activity-dependent manner. Neuroscience 180:322–333
Bone I (2007) The increasing importance of inflammation in neurological disease. Curr Opin Neurol 20:331–333
McMahon SB, Malcangio M (2009) Current challenges in glia-pain biology. Neuron 64:46–54
Wodarski R, Clark AK, Grist J, Marchand F, Malcangio M (2009) Gabapentin reverses microglial activation in the spinal cord of streptozotocin-induced diabetic rats. Eur J Pain 13:807–811
Sun S, Chen D, Lin F, Chen M, Yu H, Hou L, Li C (2016) Role of interleukin-4, the chemokine CCL3 and its receptor CCR5 in neuropathic pain. Mol Immunol 77:184–192
Tiwari V, Guan Y, Raja SN (2014) Modulating the delicate glial-neuronal interactions in neuropathic pain: promises and potential caveats. Neurosci Biobehav Rev 45:19–27
Hu XM, Liu YN, Zhang HL, Cao SB, Zhang T, Chen LP, Shen W (2015) CXCL12/CXCR4 chemokine signaling in spinal glia induces pain hypersensitivity through MAPKs-mediated neuroinflammation in bone cancer rats. J Neurochem 132:452–463
Zhang X, Zeng L, Yu T, Xu Y, Pu S, Du D, Jiang W (2014) Positive feedback loop of autocrine BDNF from microglia causes prolonged microglia activation. Cell Physiol Biochem 34:715–723
Murphy PG, Ramer MS, Borthwick L, Gauldie J, Richardson PM, Bisby MA (1999) Endogenous interleukin-6 contributes to hypersensitivity to cutaneous stimuli and changes in neuropeptides associated with chronic nerve constriction in mice. Eur J Neurosci 11:2243–2253
Mondal S, Dirks P, Rutka JT (2010) Immunolocalization of fascin, an actin-bundling protein and glial fibrillary acidic protein in human astrocytoma cells. Brain Pathol 20:190–199
Ma Y, Li A, Faller WJ, Libertini S, Fiorito F, Gillespie DA, Sansom OJ, Yamashiro S, Machesky LM (2013) Fascin 1 is transiently expressed in mouse melanoblasts during development and promotes migration and proliferation. Development 140:2203–2211
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (Nos. 81401365); a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD); Nantong science and technology project (MS12015056).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics Approval
All procedures performed in studies involving animals were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Nantong University.
Additional information
Binbin Wang, Bingbing Fan, Zhongling Xu and Xiaojuan Liu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, B., Fan, B., Dai, Q. et al. Fascin-1 Contributes to Neuropathic Pain by Promoting Inflammation in Rat Spinal Cord. Neurochem Res 43, 287–296 (2018). https://doi.org/10.1007/s11064-017-2420-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2420-8