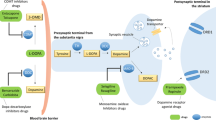
Abstract
Cytochrome P450 (CYP) 2D6 is one of the most highly active, oxidative and polymorphic enzymes known to metabolize Parkinsonian toxins and clinically established anti-Parkinson’s disease (PD) drugs. Albeit CYP2D6 gene is not present in rodents, its orthologs perform almost the similar function with imprecise substrate and inhibitor specificity. CYP2D6 expression and catalytic activity are found to be regulated at every stage of the central dogma except replication as well as at the epigenetic level. CYP2D6 gene codes for a set of alternate splice variants that give rise to a range of enzymes possessing variable catalytic activity. Case-control studies, meta-analysis and systemic reviews covering CYP2D6 polymorphism and PD risk have demonstrated that poor metabolizer phenotype possesses a considerable genetic susceptibility. Besides, ultra-rapid metabolizer offers protection against the risk in some populations while lack of positive or inverse association is also reported in other inhabitants. CYP2D6 polymorphisms resulting into deviant protein products with differing catalytic activity could lead to inter-individual variations, which could be explained to certain extent on the basis of sample size, life style factors, food habits, ethnicity and tools used for statistical analysis across various studies. Current article describes the role played by polymorphic CYP2D6 in the metabolism of anti-PD drugs/Parkinsonian toxins and how polymorphisms determine PD risk or protection. Moreover, CYP2D6 orthologs and their roles in rodent models of Parkinsonism have also been mentioned. Finally, a perspective on inconsistency in the findings and futuristic relevance of CYP2D6 polymorphisms in disease diagnosis and treatment has also been highlighted.

Similar content being viewed by others
References
Gupta SP, Kamal R, Mishra SK, Singh MK, Shukla R, Singh MP (2016) Association of polymorphism of neuronal nitric oxide synthase gene with risk to Parkinson’s disease. Mol Neurobiol 53(5):3309–3314
Kwakye GF, McMinimy RA, Aschner M (2017) Disease-toxicant interactions in Parkinson’s disease neuropathology. Neurochem Res 42(6):1772–1786
ur Rasheed MS, Tripathi MK, Mishra AK, Shukla S, Singh MP (2016) Resveratrol protects from toxin-induced Parkinsonism: plethora of proofs hitherto petty translational value. Mol Neurobiol 53(5):2751–2760
Singhal NK, Srivastava G, Agrawal S, Jain SK, Singh MP (2012) Melatonin as a neuroprotective agent in the rodent models of Parkinson’s disease: is it all set to irrefutable clinical translation? Mol Neurobiol 45(1):186–199
Franceschi M, Camerlingo M, Perego L, Bottacchi E, Truci G, Mamoli A (1988) Tuberoinfundibular dopaminergic function in Parkinson’s disease. Eur Neurol 28(3):117–119
Schober A (2004) Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res 318(1):215–224
Yadav S, Dixit A, Agrawal S, Singh A, Srivastava G, Singh AK, Srivastava PK, Prakash O, Singh MP (2012) Rodent models and contemporary molecular techniques: notable feats yet incomplete explanations of Parkinson’s disease pathogenesis. Mol Neurobiol 46(2):495–512
Mishra AK, ur Rasheed MS, Shukla S, Tripathi MK, Dixit A, Singh MP (2015) Aberrant autophagy and parkinsonism: does correction rescue from disease progression? Mol Neurobiol 51(3):893–908
Eichelbaum M, Baur MP, Dengler HJ, Osikowska-Evers BO, Tieves G, Zekorn C, Rittner C (1987) Chromosomal assignment of human cytochrome P-450 (debrisoquine/sparteine type) to chromosome 22. Br J Clin Pharmacol 23(4):455–458
Gough AC, Smith CA, Howell SM, Wolf CR, Bryant SP, Spurr NK (1993) Localization of the CYP2D gene locus to human chromosome 22q13.1 by polymerase chain reaction, in situ hybridization, and linkage analysis. Genomics 15(2):430–432
Miksys S, Rao Y, Hoffmann E, Mash DC, Tyndale RF (2002) Regional and cellular expression of CYP2D6 in human brain: higher levels in alcoholics. J Neurochem 82(6):1376–1387
Mann A, Tyndale RF (2010) Cytochrome P450 2D6 enzyme neuroprotects against 1-methyl-4-phenylpyridinium toxicity in SH-SY5Y neuronal cells. Eur J Neurosci 31(7):1185–1193
Wang X, Li J, Dong G, Yue J (2014) The endogenous substrates of brain CYP2D. Eur J Pharmacol 724:211–218
Wang B, Yang LP, Zhang XZ, Huang SQ, Bartlam M, Zhou SF (2009) New insights into the structural characteristics and functional relevance of the human cytochrome P450 2D6 enzyme. Drug Metab Rev 41(4):573–643
Bertilsson L, Dahl ML, Dalén P, Al-Shurbaji A (2002) Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol 53(2):111–122
Mann A, Miksys SL, Gaedigk A, Kish SJ, Mash DC, Tyndale RF (2012) The neuroprotective enzyme CYP2D6 increases in the brain with age and is lower in Parkinson’s disease patients. Neurobiol Aging 33(9):2160–2171
Singh S, Singh K, Patel DK, Singh C, Nath C, Singh VK, Singh RK, Singh MP (2009) The expression of CYP2D22, an ortholog of human CYP2D6, in mouse striatum and its modulation in 1-methyl 4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease phenotype and nicotine-mediated neuroprotection. Rejuvenat Res 12(3):185–197
Srivastava G, Dixit A, Yadav S, Patel DK, Prakash O, Singh MP (2012) Resveratrol potentiates cytochrome P450 2 d22-mediated neuroprotection in maneb- and paraquat-induced parkinsonism in the mouse. Free Radic Biol Med 52(8):1294–1306
Yue J, Miksys S, Hoffmann E, Tyndale RF (2008) Chronic nicotine treatment induces rat CYP2D in the brain but not in the liver: an investigation of induction and time course. J Psychiatry Neurosci 33(1):54–63
He ZX, Chen XW, Zhou ZW, Zhou SF (2015) Impact of physiological, pathological and environmental factors on the expression and activity of human cytochrome P450 2D6 and implications in precision medicine. Drug Metab Rev 47(4):470–519
Gaedigk A, Bradford LD, Marcucci KA, Leeder JS (2002) Unique CYP2D6 activity distribution and genotype-phenotype discordance in black Americans. Clin Pharmacol Ther 72(1):76–89
McLellan RA, Oscarson M, Seidegård J, Evans DA, Ingelman-Sundberg M (1997) Frequent occurrence of CYP2D6 gene duplication in Saudi Arabians. Pharmacogenetics 7(3):187–191
Bradford LD (2002) CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 3(2):229–243
Uehara S, Uno Y, Inoue T, Murayama N, Shimizu M, Sasaki E, Yamazaki H (2015) Activation and deactivation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by cytochrome P450 enzymes and flavin-containing monooxygenases in common marmosets (Callithrix jacchus). Drug Metab Dispos 43(5):735–742
Bajpai P, Sangar MC, Singh S, Tang W, Bansal S, Chowdhury G, Cheng Q, Fang JK, Martin MV, Guengerich FP, Avadhani NG (2013) Metabolism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by mitochondrion-targeted cytochrome P450 2D6: implications in Parkinson disease. J Biol Chem 288(6):4436–4451
Kimura S, Umeno M, Skoda RC, Meyer UA, Gonzalez FJ (1989) The human debrisoquine 4-hydroxylase (CYP2D) locus: sequence and identification of the polymorphic CYP2D6 gene, a related gene, and a pseudogene. Am J Hum Genet 45(6):889–904
Ingelman-Sundberg M (2005) Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenom J 5(1):6–13
Rowland P, Blaney FE, Smyth MG, Jones JJ, Leydon VR, Oxbrow AK, Lewis CJ, Tennant MG, Modi S, Eggleston DS, Chenery RJ, Bridges AM (2006) Crystal structure of human cytochrome P450 2D6. J Biol Chem 281:7614–7622
Wang A, Stout CD, Zhang Q, Johnson EF (2015) Contributions of ionic interactions and protein dynamics to cytochrome P450 2D6 (CYP2D6) substrate and inhibitor binding.. J Biol Chem 290(8):5092–5104
Kagimoto M, Heim M, Kagimoto K, Zeugin T, Meyer UA (1990) Multiple mutations of the human cytochrome P450IID6 gene (CYP2D6) in poor metabolizers of debrisoquine. Study of the functional significance of individual mutations by expression of chimeric genes. J Biol Chem 265(28):17209–17214
Lucotte G, Turpin JC, Gérard N, Panserat S, Krishnamoorthy R (1996) Mutation frequencies of the cytochrome CYP2D6 gene in Parkinson disease patients and in families. Am J Med Genet 67(4):361–365
Lu Y, Mo C, Zeng Z, Chen S, Xie Y, Peng Q, He Y, Deng Y, Wang J, Xie L, Zeng J, Li S, Qin X (2013) CYP2D6*4 allele polymorphism increases the risk of Parkinson’s disease: evidence from meta-analysis. PLoS ONE 8(12):e84413
Pang CP, Zhang J, Woo J, Chan D, Law LK, Tong SF, Kwok T, Kay R (1998) Rarity of debrisoquine hydroxylase gene polymorphism in Chinese patients with Parkinson’s disease. Mov Disord 13(3):529–532
Tyndale R, Aoyama T, Broly F, Matsunaga T, Inaba T, Kalow W, Gelboin HV, Meyer UA, Gonzalez FJ (1991) Identification of a new variant CYP2D6 allele lacking the codon encoding Lys-281: possible association with the poor metabolizer phenotype. Pharmacogenetics 1(1):26–32
Gaedigk A, Blum M, Gaedigk R, Eichelbaum M, Meyer UA (1991) Deletion of the entire cytochrome P450 CYP2D6 gene as a cause of impaired drug metabolism in poor metabolizers of the debrisoquine/sparteine polymorphism. Am J Hum Genet 48(5):943–950
Armstrong M, Daly AK, Cholerton S, Bateman DN, Idle JR (1992) Mutant debrisoquine hydroxylation genes in Parkinson’s disease. Lancet 339(8800):1017–1018
Saxena R, Shaw GL, Relling MV, Frame JN, Moir DT, Evans WE, Caporaso N, Weiffenbach B (1994) Identification of a new variant CYP2D6 allele with a single base deletion in exon 3 and its association with the poor metabolizer phenotype. Hum Mol Genet 3(6):923–926
Sachse C, Brockmöller J, Bauer S, Roots I (1997) Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet 60(2):284–295
Singh M, Khanna VK, Shukla R, Parmar D (2010) Association of polymorphism in cytochrome P450 2D6 and N-acetyltransferase-2 with Parkinson’s disease. Dis Markers 28(2):87–93
Johansson I, Lundqvist E, Bertilsson L, Dahl ML, Sjöqvist F, Ingelman-Sundberg M (1993) Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proc Natl Acad Sci USA 90(24):11825–11829
Tsuneoka Y, Matsuo Y, Iwahashi K, Takeuchi H, Ichikawa Y (1993) A novel cytochrome P-450IID6 mutant gene associated with Parkinson’s disease. J Biochem 114(2):263–266
Riedl AG, Watts PM, Jenner P, Marsden CD (1998) P450 enzymes and Parkinson’s disease: the story so far. Mov Disord 13(2):212–220
Panserat S, Sica L, Gérard N, Mathieu H, Jacqz-Aigrain E, Krishnamoorthy R (1999) CYP2D6 polymorphism in a Gabonese population: contribution of the CYP2D6*2 and CYP2D6*17 alleles to the high prevalence of the intermediate metabolic phenotype. Br J Clin Pharmacol 47(1):121–124
Ho SL, Kung MH, Li LS, Lauder IJ, Ramsden DB (1999) Cytochrome P4502D6 (debrisoquine 4-hydroxylase) and Parkinson’s disease in Chinese and Caucasians. Eur J Neurol 6(3):323–329
Lu Y, Peng Q, Zeng Z, Wang J, Deng Y, Xie L, Mo C, Zeng J, Qin X, Li S (2014) CYP2D6 phenotypes and Parkinson’s disease risk: a meta-analysis. J Neurol Sci 336(1–2):161–168
Payami H, Lee N, Zareparsi S, McNeal MG, Camicioli R, Bird TD, Sexton G, Gancher S, Kaye J, Calhoun D, Swanson PD, Nutt J (2001) Parkinson’s disease, CYP2D6 polymorphism, and age. Neurology 56:1363–1370
Elbaz A, Levecque C, Clavel J, Vidal JS, Richard F, Amouyel P, Alperovitch A, Chartier-Harlin MC, Tzourio C (2004) CYP2D6 polymorphism, pesticide exposure, and Parkinson’s disease. Ann Neurol 55:430–434
Durić G, Svetel M, Nikolaevic SI, Dragadević N, Gavrilović J, Kostić VS (2007) Polymorphisms in the genes of cytochrome oxidase P450 2D6 (CYP2D6), paraoxonase 1 (PON1) and apolipoprotein E (APOE) as risk factors for Parkinson’s disease. Vojnosanit Pregl 64(1):25–30
Deng Y, Newman B, Dunne MP, Silburn PA, Mellick GD (2004) Further evidence that interactions between CYP2D6 and pesticide exposure increase risk for Parkinson’s disease. Ann Neurol 55(6):897
Vilar R, Coelho H, Rodrigues E, Gama MJ, Rivera I, Taioli E, Lechner MC (2007) Association of A313 G polymorphism (GSTP1*B) in the glutathione-S-transferase P1 gene with sporadic Parkinson’s disease. Eur J Neurol 14(2):156–161
Caporaso NE, Lerman C, Audrain J, Boyd NR, Main D, Issaq HJ, Utermahlan B, Falk RT, Shields P (2001) Nicotine metabolism and CYP2D6 phenotype in smokers. Cancer Epidemiol Biomarkers Prev 10(3):261–263
De Palma G, Dick FD, Calzetti S, Scott NW, Prescott GJ, Osborne A, Haites N, Mozzoni P, Negrotti A, Scaglioni A, Mutti A, Geoparkinson Study Group (2010) A case-control study of Parkinson’s disease and tobacco use: gene-tobacco interactions. Mov Disord 25(7):912–919
Hiroi T, Imaoka S, Funae Y (1998) Dopamine formation from tyramine by CYP2D6. Biochem Biophys Res Commun 249(3):838–843
Mann A, Miksys S, Lee A, Mash DC, Tyndale RF (2008) Induction of the drug metabolizing enzyme CYP2D in monkey brain by chronic nicotine treatment. Neuropharmacology 55(7):1147–1155
Halling J, Petersen MS, Grandjean P, Weihe P, Brosen K (2008) Genetic predisposition to Parkinson’s disease CYP2D6 and HFE in the Faroe Islands. Pharmacogenet Genom 18(3):209–212
Gołab-Janowska M, Honczarenko K, Gawrońska-Szklarz B, Potemkowski A (2007) CYP2D6 gene polymorphism as a probable risk factor for Alzheimer’s disease and Parkinson’s disease with dementia. Neurol Neurochir Pol 41(2):113–121
Anwarullah, Aslam M, Badshah M, Abbasi R, Sultan A, Khan K, Ahmad N, von Engelhardt J (2017) Further evidence for the association of CYP2D6*4 gene polymorphism with Parkinson’s disease: a case control study. Genes Environ 39:18
Corrigan FM, Murray L, Wyatt CL, Shore RF (1998) Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson’s disease. Exp Neurol 150:339–342
Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D (2000) Organochlorine insecticides in substantia nigra in Parkinson’s disease. J Toxicol Environ Health A 59:229–234
Wang A, Cockburn M, Ly TT, Bronstein JM, Ritz B (2014) The association between ambient exposure to organophosphates and Parkinson’s disease risk. Occup Environ Med 71(4):275–281
Nandipati S, Litvan I (2016) Environmental exposures and Parkinson’s disease. Int J Environ Res Public Health 13:881
Singh AK, Tiwari MN, Upadhyay G, Patel DK, Singh D, Prakash O, Singh MP (2012) Long term exposure to cypermethrin induces nigrostriatal dopaminergic neurodegeneration in adult rats: postnatal exposure enhances the susceptibility during adulthood. Neurobiol Aging 33(2):404–415
Sams C, Mason HJ, Rawbone R (2000) Evidence for the activation of organophosphate pesticides by cytochromes P450 3A4 and 2D6 in human liver microsomes. Toxicol Lett 116(3):217–221
Costa C, Catania S, Silvari V (2003) Genotoxicity and activation of organophosphate and carbamate pesticides by cytochrome P450 2D6. G Ital Med Lav Ergon 25S(3):81–82
Hiroi T, Chow T, Imaoka S, Funae Y (2002) Catalytic specificity of CYP2D isoforms in rat and human. Drug Metabol Despos 30(9):970–976
Ravindranath V, Kommaddi RP, Pai HV (2006) Unique cytochromes P450 in human brain: implication in disease pathogenesis. J Neural Transm Suppl 70:167–171
Miksys SL, Cheung C, Gonzalez FJ, Tyndale RF (2005) Human CYP2D6 and mouse CYP2Ds: organ distribution in a humanized mouse model. Drug Metab Dispos 33(10):1495–1502
Abraham BK, Adithan C (2001) Genetic polymorphism of CYP2D6. Indian J Pharmacol 33:147–169
Acknowledgements
Authors earnestly acknowledge the Department of Science and Technology, India and Council of Scientific and Industrial Research, India, respectively, for providing research fellowship to Mohd Sami ur Rasheed and Abhishek Kumar Mishra. The CSIR-IITR communication number of this article is 3454.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors state that they do not have any conflict of interest.
Rights and permissions
About this article
Cite this article
ur Rasheed, M.S., Mishra, A.K. & Singh, M.P. Cytochrome P450 2D6 and Parkinson’s Disease: Polymorphism, Metabolic Role, Risk and Protection. Neurochem Res 42, 3353–3361 (2017). https://doi.org/10.1007/s11064-017-2384-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2384-8