Abstract
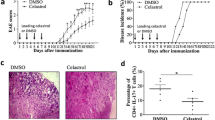
Rapamycin is a new immunosuppressant that has a primarily anti-inflammatory effect and selectively inhibits the activation of T helper (Th)-cell subsets. It is widely used to treat autoimmune disease. We studied the mechanism of rapamycin action against experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice, a classic animal model of multiple sclerosis. Rapamycin significantly inhibited the development of EAE by decreasing both clinical scores and inflammatory cell infiltration into the spinal cord. Furthermore, rapamycin reversed EAE symptoms in mice showing the initial signs of paralysis. Rapamycin, is a mammalian target of rapamycin (mTOR) inhibitor. By measuring the downstream markers phospho-mTOR (p-mTOR)/mTOR and phospho-signal transducer and activator of transcription 3 (p-STAT3)/STAT3, we showed that rapamycin suppressed the mTOR-STAT3 pathway in EAE mice. The mTOR-STAT3 signaling pathway is important for Th1 and Th17 cell responses. We found that rapamycin-treated mice had reduced proportions of Th1 and Th17 cells, as well as lower mRNA expression for the transcription factors T-bet and RoRγt in EAE mouse splenocytes. To evaluate Th1 and Th17 cell function, we examined expression of their specific cytokines in the peripheral immune system and central nervous system. Rapamycin treatment reduced protein and mRNA levels of interferon (IFN)-γand interleukin (IL)-17 in splenocytes, and reduced IFN-γ and IL-17 mRNA levels in the spinal cords of EAE mice. These findings suggest that rapamycin treatment inhibits the mTOR-STAT3 pathway in EAE mice, thereby promoting immunosuppression. This study may provide new insight into the mechanism controlling rapamycin effects in multiple sclerosis.






Similar content being viewed by others
References
Neuhaus O, Kieseier BC, Hartung HP (2007) Immunosuppressive agents in multiple sclerosis. Neurotherapeutics 4(4):654–660
Dello RC, Lisi L, Feinstein DL, Navarra P (2013) mTOR kinase, a key player in the regulation of glial functions: relevance for the therapy of multiple sclerosis. Glia 61(3):301–311
Esposito M, Ruffini F, Bellone M et al (2010) Rapamycin inhibits relapsing experimental autoimmune encephalomyelitis by both effector and regulatory T cells modulation. J Neuroimmunol 220(1–2):52–63
Lisi L, Navarra P, Cirocchi R et al (2012) Rapamycin reduces clinical signs and neuropathic pain in a chronic model of experimental autoimmune encephalomyelitis. J Neuroimmunol 243(1–2):43–51
Donia M, Mangano K, Amoroso A et al (2009) Treatment with rapamycin ameliorates clinical and histological signs of protracted relapsing experimental allergic encephalomyelitis in Dark Agouti rats and induces expansion of peripheral CD4 + CD25 + Foxp3 + regulatory T cells. J Autoimmun 33(2):135–140
Zhao YG, Wang Y, Guo Z et al (2012) Dihydroartemisinin ameliorates inflammatory disease by its reciprocal effects on Th and regulatory T cell function via modulating the mammalian target of rapamycin pathway. J Immunol 189(9):4417–4425
Koga T, Hedrich CM, Mizui M et al (2014) CaMK4-dependent activation of AKT/mTOR and CREM-alpha underlies autoimmunity-associated Th17 imbalance. J Clin Invest 124(5):2234–2245
Hou H, Cao R, Miao J et al (2016) Fingolimod ameliorates the development of experimental autoimmune encephalomyelitis by inhibiting Akt-mTOR axis in mice. Int Immunopharmacol 30:171–178
Xu Y, Li Z, Yin Y et al (2015) Ghrelin inhibits the differentiation of T helper 17 cells through mTOR/STAT3 signaling pathway. PLoS One 10(2):e0117081
Wu X, Dou Y, Yang Y et al (2015) Arctigenin exerts anti-colitis efficacy through inhibiting the differentiation of Th1 and Th17 cells via an mTORC1-dependent pathway. Biochem Pharmacol 96(4):323–336
Bielekova B, Goodwin B, Richert N et al (2000) Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med 6(10):1167–1175
Lock C, Hermans G, Pedotti R et al (2002) Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 8(5):500–508
Berard JL, Wolak K, Fournier S, David S (2010) Characterization of relapsing-remitting and chronic forms of experimental autoimmune encephalomyelitis in C57BL/6 mice. Glia 58(4):434–445
Murphy AC, Lalor SJ, Lynch MA, Mills KH (2010) Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun 24(4):641–651
Zhang GX, Gran B, Yu S et al (2003) Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol 170(4):2153–2160
Zhang F, Yang J, Jiang H, Han S (2014) An alphanubeta3 integrin-binding peptide ameliorates symptoms of chronic progressive experimental autoimmune encephalomyelitis by alleviating neuroinflammatory responses in mice. J Neuroimmune Pharmacol 9(3):399–412
Chen C, Liu Y, Liu Y, Zheng P (2010) Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice. J Clin Invest 120(11):4091–4101
Winbanks CE, Grimwood L, Gasser A, Darby IA, Hewitson TD, Becker GJ (2007) Role of the phosphatidylinositol 3-kinase and mTOR pathways in the regulation of renal fibroblast function and differentiation. Int J Biochem Cell Biol 39(1):206–219
Yokogami K, Wakisaka S, Avruch J, Reeves SA (2000) Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol 10(1):47–50
Zhou J, Wulfkuhle J, Zhang H et al (2007) Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci USA 104(41):16158–16163
Yokoyama T, Kondo Y, Kondo S (2007) Roles of mTOR and STAT3 in autophagy induced by telomere 3′ overhang-specific DNA oligonucleotides. Autophagy 3(5):496–498
Hong SM, Park CW, Cha HJ et al (2013) Rapamycin inhibits both motility through down-regulation of p-STAT3 (S727) by disrupting the mTORC2 assembly and peritoneal dissemination in sarcomatoid cholangiocarcinoma. Clin Exp Metastasis 30(2):177–187
Liu G, Yang K, Burns S, Shrestha S, Chi H (2010) The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol 11(11):1047–1056
Delgoffe GM, Kole TP, Zheng Y et al (2009) The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30(6):832–844
Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH (2000) A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100(6):655–669
Durant L, Watford WT, Ramos HL et al (2010) Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity 32(5):605–615
Wing AC, Hygino J, Ferreira TB, et al (2016) Interleukin-17- and interleukin-22-secreting myelin-specific CD4(+) T cells resistant to corticoids are related with active brain lesions in multiple sclerosis patients. Immunology 147(2):212–220
Zhang J, Zeng YQ, Zhang J et al (2015) Tripchlorolide ameliorates experimental autoimmune encephalomyelitis by down-regulating ERK1/2-NF-kappaB and JAK/STAT signaling pathways. J Neurochem 133(1):104–112
Traugott U, Lebon P (1988) Interferon-gamma and Ia antigen are present on astrocytes in active chronic multiple sclerosis lesions. J Neurol Sci 84(2–3):257–264
Pelfrey CM, Rudick RA, Cotleur AC, Lee JC, Tary-Lehmann M, Lehmann PV (2000) Quantification of self-recognition in multiple sclerosis by single-cell analysis of cytokine production. J Immunol 165(3):1641–1651
Lees JR, Golumbek PT, Sim J, Dorsey D, Russell JH (2008) Regional CNS responses to IFN-gamma determine lesion localization patterns during EAE pathogenesis. J Exp Med.. 205(11):2633–2642
Babaloo Z, Aliparasti MR, Babaiea F, Almasi S, Baradaran B, Farhoudi M (2015) The role of Th17 cells in patients with relapsing-remitting multiple sclerosis: interleukin-17 A and interleukin-17F serum levels. Immunol Lett 164(2):76–80
Tang SC, Fan XH, Pan QM, Sun QS, Liu Y (2015) Decreased expression of IL-27 and its correlation with Th1 and Th17 cells in progressive multiple sclerosis. J Neurol Sci 348(1–2):174–180
Sun Y, Tian T, Gao J et al (2016) Metformin ameliorates the development of experimental autoimmune encephalomyelitis by regulating T helper 17 and regulatory T cells in mice. J Neuroimmunol 292:58–67
Li B, Cui W, Liu J et al (2013) Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Exp Neurol 250:239–249
Zhang J, Cheng Y, Cui W, Li M, Li B, Guo L (2014) MicroRNA-155 modulates Th1 and Th17 cell differentiation and is associated with multiple sclerosis and experimental autoimmune encephalomyelitis. J Neuroimmunol 266(1–2):56–63
Xiao J, Yang R, Yang L, Fan X, Liu W, Deng W (2015) Kirenol attenuates experimental autoimmune encephalomyelitis by inhibiting differentiation of Th1 and th17 cells and inducing apoptosis of effector T cells. Sci Rep 5:9022
Shen R, Deng W, Li C, Zeng G (2015) A natural flavonoid glucoside icariin inhibits Th1 and Th17 cell differentiation and ameliorates experimental autoimmune encephalomyelitis. Int Immunopharmacol 24(2):224–231
Togha M, Jahanshahi M, Alizadeh L et al (2016) Rapamycin augments immunomodulatory properties of bone marrow-derived mesenchymal stem cells in experimental autoimmune encephalomyelitis. Mol Neurobiol. doi:10.1007/s12035-016-9840-3
Acknowledgments
We would like to thank Chunyan Li, Jingci Yang, Yansu Guo, Dongxia Wu and Hongran Wu for expert technical assistances. Financial support was obtained from the National Natural Science Foundation of China (No. 81100884) and the key project of Medical Science Research in Hebei Province (No. 20150213).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Hou, H., Miao, J., Cao, R. et al. Rapamycin Ameliorates Experimental Autoimmune Encephalomyelitis by Suppressing the mTOR-STAT3 Pathway. Neurochem Res 42, 2831–2840 (2017). https://doi.org/10.1007/s11064-017-2296-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2296-7