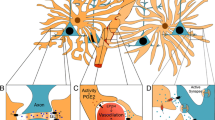
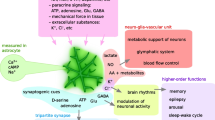
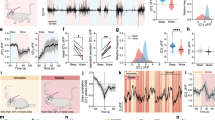
Abstract
We continuously need to adapt to changing conditions within our surrounding environment, and our brain needs to quickly shift between resting and working activity states in order to allow appropriate behaviors. These global state shifts are intimately linked to the brain-wide release of the neuromodulators, noradrenaline and acetylcholine. Astrocytes have emerged as a new player participating in the regulation of brain activity, and have recently been implicated in brain state shifts. Astrocytes display global Ca2+ signaling in response to activation of the noradrenergic system, but whether astrocytic Ca2+ signaling is causative or correlative for shifts in brain state and neural activity patterns is not known. Here we review the current available literature on astrocytic Ca2+ signaling in awake animals in order to explore the role of astrocytic signaling in brain state shifts. Furthermore, we look at the development and availability of innovative new methodological tools that are opening up for new ways of visualizing and perturbing astrocyte activity in awake behaving animals. With these new tools at hand, the field of astrocyte research will likely be able to elucidate the causal and mechanistic roles of astrocytes in complex behaviors within a very near future.



Similar content being viewed by others
References
Harris KD, Thiele A (2011) Cortical state and attention. Nat Rev Neurosci 12:509–523. doi:10.1038/nrn3084
Lee S-H, Dan Y (2012) Neuromodulation of brain states. Neuron 76:209–222. doi:10.1016/j.neuron.2012.09.012
McGinley MJ, David SV, McCormick DA (2015) Cortical membrane potential signature of optimal states for sensory signal detection. Neuron 87:179–192. doi:10.1016/j.neuron.2015.05.038
Niell CM, Stryker MP (2010) Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65:472–479. doi:10.1016/j.neuron.2010.01.033
Schneider DM, Nelson A, Mooney R (2014) A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 513:189–194. doi:10.1038/nature13724
Vinck M, Batista-Brito R, Knoblich U, Cardin JA (2015) Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86:740–754. doi:10.1016/j.neuron.2015.03.028
Ding F, ODonnell J, Xu Q et al (2016) Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 352:550–555. doi:10.1126/science.aad4821
McCormick DA, Bal T (1997) Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci 20:185–215. doi:10.1146/annurev.neuro.20.1.185
Steriade M, McCormick DA, Sejnowski TJ (1993) Thalamocortical oscillations in the sleeping and aroused brain. Science 262:679–685. doi:10.1126/science.8235588
Steriade M, Amzica F, Nuñez A (1993) Cholinergic and noradrenergic modulation of the slow (approximately 0.3 Hz) oscillation in neocortical cells. J Neurophysiol 70:1385–1400
Polack P-O, Friedman J, Golshani P (2013) Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci 16:1331–1339. doi:10.1038/nn.3464
Yamashita T, Pala A, Pedrido L et al (2013) Membrane potential dynamics of neocortical projection neurons driving target-specific signals. Neuron 80:1477–1490. doi:10.1016/j.neuron.2013.10.059
Schiemann J, Puggioni P, Dacre J et al (2015) Cellular mechanisms underlying behavioral state-dependent bidirectional modulation of motor cortex output. Cell Rep 11: 1319–1330. doi:10.1016/j.celrep.2015.04.042
Fu Y, Tucciarone JM, Espinosa JS et al (2014) A cortical circuit for gain control by behavioral state. Cell 156:1139–1152. doi:10.1016/j.cell.2014.01.050
Mineault PJ, Tring E, Trachtenberg JT, Ringach DL (2016) Enhanced spatial resolution during locomotion and heightened attention in mouse primary visual cortex. J Neurosci 36:6382–6392. doi:10.1523/JNEUROSCI.0430-16.2016
McGinley MJ, Vinck M, Reimer J et al (2015) Waking state: rapid variations modulate neural and behavioral responses. Neuron 87:1143–1161. doi:10.1016/j.neuron.2015.09.012
Bennett C, Arroyo S, Hestrin S (2013) Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron 80:350–357. doi:10.1016/j.neuron.2013.08.007
Hawkins A, Olszewski J (1957) Glia/nerve cell index for cortex of the whale. Science 126:76–77
Herculano-Houzel S (2014) The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia 62:1377–1391. doi:10.1002/glia.22683
Khakh BS, Sofroniew MV (2015) Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18:942–952. doi:10.1038/nn.4043
Amzica F, Neckelmann DAG (1999) Membrane capacitance of cortical neurons and glia during sleep oscillations and spike-wave seizures. J Neurophysiol 82:2731–2746
Amzica F (2002) In vivo electrophysiological evidences for cortical neuron–glia interactions during slow (<1 Hz) and paroxysmal sleep oscillations. J Physiol 96:209–219
Poskanzer KE, Yuste R (2011) Astrocytic regulation of cortical UP states. Proc Natl Acad Sci 108:18453–18458. doi:10.1073/pnas.1112378108
Poskanzer KE, Yuste R (2016) Astrocytes regulate cortical state switching in vivo. Proc Natl Acad Sci USA 113:E2675–E2684. doi:10.1073/pnas.1520759113
Wang X, Lou N, Xu Q et al (2006) Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci 9:816–823. doi:10.1038/nn1703
Paukert M, Agarwal A, Cha J et al (2014) Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82:1263–1270. doi:10.1016/j.neuron.2014.04.038
Eggermann E, Kremer Y, Crochet S, Petersen CCH (2014) Cholinergic signals in mouse barrel cortex during active whisker sensing. Cell Rep 9:1654–1661. doi:10.1016/j.celrep.2014.11.005
Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450. doi:10.1146/annurev.neuro.28.061604.135709
Mircea S, Robert MW (2005) Brain control of Wakefulness and sleep. Vasa. doi:10.1007/b102230
Armstrong-James M, Fox K (1983) Effects of ionophoresed noradrenaline on the spontaneous activity of neurones in rat primary somatosensory cortex. J Physiol 335:427–447. doi:10.1113/jphysiol.1983.sp014542
Videen TO, Daw NW, Rader RK (1984) The effect of norepinephrine on visual cortical neurons in kittens and adult cats. J Neurosci 4:1607–1617
Bevan P, Bradshaw CM, Szabadi E (1977) The pharmacology of adrenergic neuronal responses in the cerebral cortex: evidence for excitatory alpha- and inhibitory beta-receptors. Br J Pharmacol 59:635–641
Bergles DE, Doze V a, Madison DV, Smith SJ (1996) Excitatory actions of norepinephrine on multiple classes of hippocampal CA1 interneurons. J Neurosci 16:572–585
McCormick DA (1992) Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol 39:337–388. doi:10.1016/0301-0082(92)90012-4
McCormick DA (1992) Cellular mechanisms underlying cholinergic and noradrenergic modulation of neuronal firing mode in the cat and guinea pig dorsal lateral geniculate nucleus. J Neurosci 12:278–289
Madison DV, Nicoll RA (1986) Cyclic adenosine 3′,5′-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J Physiol 372:245–259. doi:10.1113/jphysiol.1986.sp016007
Madison DV, Nicoll RA (1986) Actions of noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. J Physiol 372:221–244
McCormick DA, Prince DA (1987) Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. J Physiol 392:147–165
McCormick DA (1989) Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci 12:215–221. doi:10.1016/0166-2236(89)90125-2
Sillito AM, Kemp JA, Berardi N (1983) The cholinergic influence on the function of the cat dorsal lateral geniculate nucleus (dLGN). Brain Res 280:299–307. doi:10.1016/0006-8993(83)90059-8
Krnjevjic K, Pumain R, Renaudt L (1971) The mechanism of excitation by acetylcholine in the cerebral cortex. J Physiol 215:247–268. doi:10.1113/jphysiol.1971.sp009467
Constanti A, Galvan M (1983) M-current in voltage-clamped olfactory cortex neurones. Neurosci Lett 39:65–70. doi:10.1016/0304-3940(83)90166-0
Constanti A, Sim JA (1987) Calcium-dependent potassium conductance in guinea-pig olfactory cortex neurones in vitro. J Physiol 387:173–194. doi:10.1113/jphysiol.1987.sp016569
Madison DV, Lancaster B, Nicoll RA (1987) Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci 7:733–741
McCormick DA, Williamson A (1989) Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci USA 86:8098–8102
Lee S, Kruglikov I, Huang ZJ et al (2013) A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci 16:1662–1670. doi:10.1038/nn.3544
Zagha E, McCormick DA (2014) Neural control of brain state. Curr Opin Neurobiol 29:178–186. doi:10.1016/j.conb.2014.09.010
Duffy S, MacVicar BA (1995) Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci 15:5535–5550
Bazargani N, Attwell D (2016) Astrocyte calcium signaling: the third wave. Nat Neurosci 19:182–189. doi:10.1038/nn.4201
Sun W, McConnell E, Pare J-F et al (2013) Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339:197–200. doi:10.1126/science.1226740.Glutamate-Dependent
Thrane AS, Rangroo Thrane V, Zeppenfeld D et al (2012) General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc Natl Acad Sci USA 109:18974–18979. doi:10.1073/pnas.1209448109
Schummers J, Yu H, Sur M (2008) Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320:1638–1643. doi:10.1126/science.1156120
Winship IR, Plaa N, Murphy TH (2007) Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci 27:6268–6272
Bekar LK, He W, Nedergaard M (2008) Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex 18:2789–2795. doi:10.1093/cercor/bhn040
Duffy S, MacVicar B (1995) Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci 15:5535–5550
Salm AK, McCarthy KD (1990) Norepinephrine-evoked calcium transients in cultured cerebral type 1 astroglia. Glia 3:529–538. doi:10.1002/glia.440030612
Shao Y, McCarthy KD (1997) Responses of Bergmann glia and granule neurons in situ to N-methyl-d-aspartate, norepinephrine, and high potassium. J Neurochem 68:2405–2411
Srinivasan R, Huang BS, Venugopal S et al (2015) Ca2+ signaling in astrocytes from Ip3r2–/– mice in brain slices and during startle responses in vivo. Nat Neurosci. doi:10.1038/nn.4001
Ding F, O’Donnell J, Thrane AS et al (2013) α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54:387–394. doi:10.1016/j.ceca.2013.09.001
Foote SL, Aston-Jones G, Bloom FE (1980) Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA 77:3033–3037
Ma Z, Stork T, Bergles DE, Freeman MR (2016) Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature 539:428–432. doi:10.1038/nature20145
Takata N, Mishima T, Hisatsune C et al (2011) Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci 31:18155–18165. doi:10.1523/JNEUROSCI.5289-11.2011
Chen N, Sugihara H, Sharma J et al (2012) Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci 109:E2832–E2841. doi:10.1073/pnas.1206557109
Shelton MK, McCarthy KD (2000) Hippocampal astrocytes exhibit Ca2+-elevating muscarinic cholinergic and histaminergic receptors in situ. J Neurochem 74:555–563
Pabst M, Braganza O, Dannenberg H et al (2016) Astrocyte intermediaries of septal cholinergic modulation in the hippocampus. Neuron 90:853–865. doi:10.1016/j.neuron.2016.04.003
Araque A, Martín ED, Perea G et al (2002) Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J Neurosci 22:2443–2450
Tellez S, Colpaert F, Marien M (1995) The alpha 2-adrenoceptor antagonist, (+)-efaroxan, enhances acetylcholine release in the rat cortex in vivo. Eur J Pharmacol 277:113–116
Aoki C, Go CG, Venkatesan C, Kurose H (1994) Perikaryal and synaptic localization of alpha 2A-adrenergic receptor-like immunoreactivity. Brain Res 650:181–204
Rao TS, Correa LD, Adams P et al (2003) Pharmacological characterization of dopamine, norepinephrine and serotonin release in the rat prefrontal cortex by neuronal nicotinic acetylcholine receptor agonists. Brain Res 990:203–208
Dombeck DA, Khabbaz AN, Collman F et al (2007) Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56:43–57. doi:10.1016/j.neuron.2007.08.003
Nimmerjahn A, Mukamel EA, Schnitzer MJ Motor behavior activates Bergmann glial. Networks. doi:10.1016/j.neuron.2009.03.019
Rasmussen R, Nedergaard M, Petersen NC (2016) Sulforhodamine 101, a widely used astrocyte marker, can induce cortical seizure-like activity at concentrations commonly used. Sci Rep 6:30433. doi:10.1038/srep30433
Dombeck DA, Graziano MS, Tank DW (2009) Functional clustering of neurons in motor cortex determined by cellular resolution imaging in awake behaving mice. J Neurosci 29:13751–13760. doi:10.1523/JNEUROSCI.2985-09.2009
Yerkes RM, Dodson JD (1908) The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol 18:459–482. doi:10.1037/h0073415
Chen F-J, Sara SJ (2007) Locus coeruleus activation by foot shock or electrical stimulation inhibits amygdala neurons. Neuroscience 144:472–481. doi:10.1016/j.neuroscience.2006.09.037
Nestler EJ, Alreja M, Aghajanian GK (1999) Molecular control of locus coeruleus neurotransmission. Biol Psychiatry 46:1131–1139
Melia KR, Rasmussen K, Terwilliger RZ et al (1992) Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase in the rat locus coeruleus: effects of chronic stress and drug treatments. J Neurochem 58:494–502
Miner LH, Jedema HP, Moore FW et al (2006) Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J Neurosci 26:1571–1578. doi:10.1523/JNEUROSCI.4450-05.2006
Fan Y, Chen P, Li Y, Zhu M-Y (2013) Effects of chronic social defeat on expression of dopamine β-hydroxylase in rat brains. Synapse 67:300–312. doi:10.1002/syn.21641
Mana MJ, Grace AA (1997) Chronic cold stress alters the basal and evoked electrophysiological activity of rat locus coeruleus neurons. Neuroscience 81:1055–1064
Weissman MM, Bland RC, Canino GJ et al (1996) Cross-national epidemiology of major depression and bipolar disorder. JAMA 276:293–299
Breslau N (2009) The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse 10:198–210. doi:10.1177/1524838009334448
Tian G-F, Azmi H, Takano T et al (2005) An astrocytic basis of epilepsy. Nat Med 11:973–981. doi:10.1038/nm1277
Chandler DJ, Gao W-J, Waterhouse BD (2014) Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc Natl Acad Sci USA 111:6816–6821. doi:10.1073/pnas.1320827111
Bellesi M, Tononi G, Cirelli C, Serra PA (2016) Region-specific dissociation between cortical noradrenaline levels and the sleep/wake cycle. Sleep 39:143–154. doi:10.5665/sleep.5336
Chandler DJ (2015) Evidence for a specialized role of the locus coeruleus noradrenergic system in cortical circuitries and behavioral operations. Brain Res. doi:10.1016/j.brainres.2015.11.022
Nuriya M, Takeuchi M, Yasui M (2016) Background norepinephrine primes astrocytic calcium responses to subsequent norepinephrine stimuli in the cerebral cortex. Biochem Biophys Res Commun. doi:10.1016/j.bbrc.2016.12.073
Devilbiss DM, Waterhouse BD (2011) Phasic and tonic patterns of locus coeruleus output differentially modulate sensory network function in the awake rat. J Neurophysiol 105:69–87. doi:10.1152/jn.00445.2010
Halassa MM, Fellin T, Takano H et al (2007) Synaptic islands defined by the territory of a single astrocyte. J Neurosci 27:6473–6477. doi:10.1523/JNEUROSCI.1419-07.2007
Bushong EA, Martone ME, Jones YZ, Ellisman MH (2002) Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22:183–192
Oberheim NA, Tian GF, Han X et al (2008) Loss of astrocytic domain organization in the epileptic brain. J Neurosci 28:3264–3276. doi:10.1523/JNEUROSCI.4980-07.2008
Haydon PG, Nedergaard M (2014) How do astrocytes participate in neural plasticity? Cold Spring Harb Perspect Biol 7:a020438. doi:10.1101/cshperspect.a020438
Witcher MR, Kirov SA, Harris KM (2007) Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia 55:13–23. doi:10.1002/glia.20415
Bernardinelli Y, Randall J, Janett E et al (2014) Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr Biol 24:1679–1688. doi:10.1016/j.cub.2014.06.025
Perez-Alvarez A, Navarrete M, Covelo A et al (2014) Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J Neurosci 34:12738–12744. doi:10.1523/JNEUROSCI.2401-14.2014
Genoud C, Quairiaux C, Steiner P et al (2006) Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol 4:e343. doi:10.1371/journal.pbio.0040343
Lushnikova I, Skibo G, Muller D, Nikonenko I (2009) Synaptic potentiation induces increased glial coverage of excitatory synapses in CA1 hippocampus. Hippocampus 19:753–762. doi:10.1002/hipo.20551
Pannasch U, Freche D, Dallérac G et al (2014) Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci 17:549–558. doi:10.1038/nn.3662
Zeng X-N, Sun X-L, Gao L et al (2007) Aquaporin-4 deficiency down-regulates glutamate uptake and GLT-1 expression in astrocytes. Mol Cell Neurosci 34:34–39. doi:10.1016/j.mcn.2006.09.008
Li Y-K, Wang F, Wang W et al (2012) Aquaporin-4 deficiency impairs synaptic plasticity and associative fear memory in the lateral amygdala: involvement of downregulation of glutamate transporter-1 expression. Neuropsychopharmacology 37:1867–1878. doi:10.1038/npp.2012.34
Shigetomi E, Tong X, Kwan KY et al (2011) TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci 15:70–80. doi:10.1038/nn.3000
Rangroo Thrane V, Thrane AS, Wang F et al (2013) Ammonia triggers neuronal disinhibition and seizures by impairing astrocyte potassium buffering. Nat Med 19:1643–1648. doi:10.1038/nm.3400
Wang F, Smith NA, Xu Q et al (2012) Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci Signal 5:ra26. doi:10.1126/scisignal.2002334
Wallraff A, Köhling R, Heinemann U et al (2006) The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci 26:5438–5447. doi:10.1523/JNEUROSCI.0037-06.2006
Seifert G, Hüttmann K, Binder DK et al (2009) Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J Neurosci 29:7474–7488. doi:10.1523/JNEUROSCI.3790-08.2009
Larsen BR, Assentoft M, Cotrina ML et al (2014) Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia 62:608–622. doi:10.1002/glia.22629
Wang F, Smith NA, Xu Q et al (2012) Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci Signal 5:ra26
Tas PW, Massa PT, Kress HG, Koschel K (1987) Characterization of an Na+/K+/Cl− co-transport in primary cultures of rat astrocytes. Biochim Biophys Acta 903:411–416
Tas PW, Massa PT, Koschel K (1986) Preliminary characterization of an Na+,K+,Cl− co-transport activity in cultured human astrocytes. Neurosci Lett 70:369–373
Xu J, Song D, Xue Z et al (2013) Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes: potential implications for K+ homeostasis and glycogen usage in brain. Neurochem Res 38:472–485. doi:10.1007/s11064-012-0938-3
Choi HB, Gordon GRJ, Zhou N et al (2012) Metabolic communication between astrocytes and neurons via bicarbonate-responsive soluble adenylyl cyclase. Neuron 75:1094–1104. doi:10.1016/j.neuron.2012.08.032
Cataldo AM, Broadwell RD (1986) Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J Neurocytol 15:511–524
Subbarao KV, Stolzenburg JU, Hertz L (1995) Pharmacological characteristics of potassium-induced, glycogenolysis in astrocytes. Neurosci Lett 196:45–48
Vardjan N, Gabrijel M, Potokar M et al (2012) IFN-γ-induced increase in the mobility of MHC class II compartments in astrocytes depends on intermediate filaments. J Neuroinflammation 9:144. doi:10.1186/1742-2094-9-144
Gharami K, Das S (2004) Delayed but sustained induction of mitogen-activated protein kinase activity is associated with beta-adrenergic receptor-mediated morphological differentiation of astrocytes. J Neurochem 88:12–22
Won CL, Oh YS (2000) cAMP-induced stellation in primary astrocyte cultures with regional heterogeneity. Brain Res 887:250–258
Prebil M, Vardjan N, Jensen J et al (2011) Dynamic monitoring of cytosolic glucose in single astrocytes. Glia 59:903–913. doi:10.1002/glia.21161
Kreft M, Bak LK, Waagepetersen HS, Schousboe A (2012) Aspects of astrocyte energy metabolism, amino acid neurotransmitter homoeostasis and metabolic compartmentation. ASN Neuro 4:187–199. doi:10.1042/AN20120007
McCormack JG, Halestrap AP, Denton RM (1990) Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 70:391–425
Griffiths EJ, Rutter GA (2009) Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim Biophys Acta 1787:1324–1333. doi:10.1016/j.bbabio.2009.01.019
Fellin T, Pascual O, Gobbo S et al (2004) Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43:729–743. doi:10.1016/j.neuron.2004.08.011
Wang F, Smith NA, Xu Q et al (2013) Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. J Neurosci 33:17404–17412. doi:10.1523/JNEUROSCI.2178-13.2013
Agulhon C, Boyt KM, Xie AX et al (2013) Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein-coupled receptor activation in vivo. J Physiol 591:5599–5609. doi:10.1113/jphysiol.2013.261289
Armbruster BN, Li X, Pausch MH et al (2007) Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 104:5163–5168. doi:10.1073/pnas.0700293104
Zhu H, Roth BL (2014) DREADD: a chemogenetic GPCR signaling platform. Int J Neuropsychopharmacol. doi:10.1093/ijnp/pyu007
Gourine AV, Kasymov V, Marina N et al (2010) Astrocytes control breathing through pH-dependent release of ATP. Science 329:571–575. doi:10.1126/science.1190721
Perea G, Yang A, Boyden ES, Sur M (2014) Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nat Commun 5:3262. doi:10.1038/ncomms4262
Yamashita A, Hamada A, Suhara Y et al (2014) Astrocytic activation in the anterior cingulate cortex is critical for sleep disorder under neuropathic pain. Synapse 68:235–247. doi:10.1002/syn.21733
Fujita T, Chen MJ, Li B et al (2014) Cellular/molecular neuronal transgene expression in dominant-negative SNARE mice. doi:10.1523/JNEUROSCI.2585-14.2014
Xie AX, Petravicz J, McCarthy KD (2015) Molecular approaches for manipulating astrocytic signaling in vivo. Front Cell Neurosci 9:144. doi:10.3389/fncel.2015.00144
Agulhon C, Fiacco TA, McCarthy KD (2010) Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327:1250–1254. doi:10.1126/science.1184821
Petravicz J, Boyt KM, McCarthy KD (2014) Astrocyte IP3R2-dependent Ca2+ signaling is not a major modulator of neuronal pathways governing behavior. Front Behav Neurosci 8:384. doi:10.3389/fnbeh.2014.00384
Haustein MD, Kracun S, Lu X-H et al (2014) Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 82:413–429. doi:10.1016/j.neuron.2014.02.041
Kanemaru K, Sekiya H, Xu M et al (2014) In vivo visualization of subtle, transient, and local activity of astrocytes using an ultrasensitive Ca(2+) indicator. Cell Rep 8:311–318. doi:10.1016/j.celrep.2014.05.056
Shigetomi E, Bushong EA, Haustein MD et al (2013) Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol 141:633–647. doi:10.1085/jgp.201210949
Wettschureck N, Rütten H, Zywietz A et al (2001) Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Galphaq/Galpha11 in cardiomyocytes. Nat Med 7:1236–1240. doi:10.1038/nm1101-1236
Monai H, Ohkura M, Tanaka M et al (2016) Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat Commun 7:11100. doi:10.1038/ncomms11100
Akerboom J, Carreras Calderón N, Tian L et al (2013) Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci 6:2. doi:10.3389/fnmol.2013.00002
Ghosh KK, Burns LD, Cocker ED et al (2011) Miniaturized integration of a fluorescence microscope. Nat Methods 8:871–878. doi:10.1038/nmeth.1694
Gunaydin LA, Grosenick L, Finkelstein JC et al (2014) Natural neural projection dynamics underlying social behavior. Cell 157:1535–1551. doi:10.1016/j.cell.2014.05.017
Lerner TN, Shilyansky C, Davidson TJ et al (2015) Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162:635–647. doi:10.1016/j.cell.2015.07.014
Sekiguchi KJ, Shekhtmeyster P, Merten K et al (2016) Imaging large-scale cellular activity in spinal cord of freely behaving mice. Nat Commun 7:11450. doi:10.1038/ncomms11450
Funding
This study was funded by the Novo Nordisk Foundation, the Office of Naval Research/Department of the Navy, JPND, and NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Celia Kjaerby and Rune Rasmussen have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kjaerby, C., Rasmussen, R., Andersen, M. et al. Does Global Astrocytic Calcium Signaling Participate in Awake Brain State Transitions and Neuronal Circuit Function?. Neurochem Res 42, 1810–1822 (2017). https://doi.org/10.1007/s11064-017-2195-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2195-y