Abstract
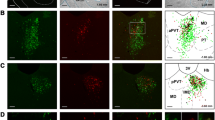
It has been suggested that the trigemino-thalamic and trigemino-parabrachial projection neurons in the medullary dorsal horn (MDH) are highly implicated in the sensory-discriminative and emotional/affective aspects of orofacial pain, respectively. In previous studies, some neurons were reported to send projections to both the thalamus and parabrachial nucleus by way of collaterals in the MDH. However, little is known about the chemoarchitecture of this group of neurons. Thus, in the present study, we determined whether the neurokinin-1 (NK-1) receptor, which is crucial for primary orofacial pain signaling, was expressed in MDH neurons co-innervating the thalamus and parabrachial nucleus. Vesicular glutamate transporter 2 (VGLUT2) mRNA, a biomarker for the subgroup of glutamatergic neurons closely related to pain sensation, was assessed in trigemino-parabrachial projection neurons in the MDH. After stereotactic injection of fluorogold (FG) and cholera toxin subunit B (CTB) into the ventral posteromedial thalamic nucleus (VPM) and parabrachial nucleus (PBN), respectively, triple labeling with fluorescence dyes for FG, CTB and NK-1 receptor (NK-1R) revealed that approximately 76 % of the total FG/CTB dually labeled neurons were detected as NK-1R-immunopositive, and more than 94 % of the triple-labeled neurons were distributed in lamina I. In addition, by FG retrograde tract-tracing combined with fluorescence in situ hybridization (FISH) for VGLUT2 mRNA, 54, 48 and 70 % of FG-labeled neurons in laminae I, II and III, respectively, of the MDH co-expressed FG and VGLUT2 mRNA. Thus, most of the MDH neurons co-innervating the thalamus and PBN were glutamatergic. Most MDH neurons providing the collateral axons to both the thalamus and parabrachial nucleus in rats were NK-1R-immunopositive and expressed VGLUT2 mRNA. NK-1R and VGLUT2 in MDH neurons may be involved in both sensory-discriminative and emotional/affective aspects of orofacial pain processing.




Similar content being viewed by others
Abbreviations
- CL:
-
Centromedial thalamic nucleus
- CM:
-
Central medial thalamic nucleus
- CTB:
-
Chorea toxin B unit
- f:
-
Fornix
- FG:
-
Fluoro-gold
- FISH:
-
Fluorescence in situ hybridization
- Hb:
-
Habenular nucleus
- ic:
-
Internal capsule
- KF:
-
Kölliker–Fuse nucleus
- LD:
-
Laterodorsal thalamic nucleus
- LPB:
-
Lateral parabrachial nucleus
- MD:
-
Mediodorsal thalamic nucleus
- MDH:
-
Medullary dorsal horn
- MPB:
-
Medial parabrachial nucleus
- NK-1:
-
Neurokinin-1
- NK-1R:
-
Neurokinin-1 receptor
- mt:
-
Mammillothalamic tract
- opt:
-
Optic tract
- Po:
-
Posterior thalamic nuclear group
- Rt:
-
Reticular thalamic nucleus
- scp:
-
Superior cerebellar peduncle
- SPR:
-
Substance P receptors
- VPL:
-
Ventral posterolateral thalamic nucleus
- VPM:
-
Ventral posteromedial thalamic nucleus
- ZI:
-
Zona incerta
- 3V:
-
Third ventricle
- 4V:
-
Fourth ventricle
- Vc:
-
Caudal subnucleus of spinal trigeminal nucleus
- VGLUTs:
-
Vesicular glutamate transporters
- VL:
-
Ventrolateral thalamic nucleus
- VM:
-
Ventromedial thalamic nucleus
References
Waite P (2004) Trigeminal sensory system. In: Paxinos G (ed) The rat nervous system. Academic Press, San Diego, pp 705–724
Bereiter DA, Hirata H, Hu JW (2000) Trigeminal subnucleus caudalis: beyond homologies with the spinal dorsal horn. Pain 88:221–224
Renehan WE, Jacquin MF, Mooney RD, Rhoades RW (1986) Structure-function relationships in rat medullary and cervical dorsal horns. II. Medullary dorsal horn cells. J Neurophysiol 55:1187–1201
Iwata K, Tashiro A, Tsuboi Y, Imai T, Sumino R, Morimoto T, Dubner R, Ren K (1999) Medullary dorsal horn neuronal activity in rats with persistent temporomandibular joint and perioral inflammation. J Neurophysiol 82:1244–1253
Dado RJ, Giesler GJ Jr (1990) Afferent input to nucleus submedius in rats: retrograde labeling of neurons in the spinal cord and caudal medulla. J Neurosci 10:2672–2686
Iwata K, Kenshalo DR Jr, Dubner R, Nahin RL (1992) Diencephalic projections from the superficial and deep laminae of the medullary dorsal horn in the rat. J Comp Neurol 321:404–420
Li YQ (1999) Substance P receptor-like immunoreactive neurons in the caudal spinal trigeminal nucleus send axons to the gelatinosus thalamic nucleus in the rat. J Hirnforsch 39:277–282
Fukushima T, Kerr FW (1979) Organization of trigeminothalamic tracts and other thalamic afferent systems of the brainstem in the rat: presence of gelatinosa neurons with thalamic connections. J Comp Neurol 183:169–184
Shigenaga Y, Takabatake M, Sugimoto T, Sakai A (1979) Neurons in marginal layer of trigeminal nucleus caudalis projecting to ventrobasal complex (VG) and posterior nuclear group (PO) demonstrated by retrograde labeling with horseradish peroxidase. Brain Res 166:391–396
Krout KE, Belzer RE, Loewy AD (2002) Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol 448:53–101
Willis WD, Westlund KN (1997) Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol 14:2–31
Slugg RM, Light AR (1994) Spinal cord and trigeminal projections to the pontine parabrachial region in the rat as demonstrated with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 339:49–61
Feil K, Herbert H (1995) Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kolliker–Fuse nuclei. J Comp Neurol 353:506–528
Allen GV, Barbrick B, Esser MJ (1996) Trigeminal-parabrachial connections: possible pathway for nociception-induced cardiovascular reflex responses. Brain Res 715:125–135
Basbaum AI, Braz. JM (2010) Transgenic mouse models for the tracing of “pain” pathways translational pain research: from mouse to man. CRC Press, Boca Raton
Cechetto DF, Standaert DG, Saper CB (1985) Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol 240:153–160
Standaert DG, Watson SJ, Houghten RA, Saper CB (1986) Opioid peptide immunoreactivity in spinal and trigeminal dorsal horn neurons projecting to the parabrachial nucleus in the rat. J Neurosci 6:1220–1226
Li J, Xiong K, Pang Y, Dong Y, Kaneko T, Mizuno N (2006) Medullary dorsal horn neurons providing axons to both the parabrachial nucleus and thalamus. J Comp Neurol 498:539–551
De Felipe C, Herrero JF, O’Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F, Hunt SP (1998) Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature 392:394–397
Molina-Ortega F, Lomas-Vega R, Hita-Contreras F, Plaza Manzano G, Achalandabaso A, Ramos-Morcillo AJ, Martinez-Amat A (2014) Immediate effects of spinal manipulation on nitric oxide, substance P and pain perception. Man Ther 19:411–417
Teodoro FC, Tronco Junior MF, Zampronio AR, Martini AC, Rae GA, Chichorro JG (2013) Peripheral substance P and neurokinin-1 receptors have a role in inflammatory and neuropathic orofacial pain models. Neuropeptides 47:199–206
Rogoz K, Andersen HH, Kullander K, Lagerstrom MC (2014) Glutamate, substance P, and calcitonin gene-related peptide cooperate in inflammation-induced heat hyperalgesia. Mol Pharmacol 85:322–334
Takeda M, Tanimoto T, Nasu M, Ikeda M, Kadoi J, Matsumoto S (2005) Activation of NK1 receptor of trigeminal root ganglion via substance P paracrine mechanism contributes to the mechanical allodynia in the temporomandibular joint inflammation in rats. Pain 116:375–385
Li JL, Wang D, Kaneko T, Shigemoto R, Nomura S, Mizuno N (2000) The relationship between neurokinin-1 receptor and substance P in the medullary dorsal horn: a light and electron microscopic immunohistochemical study in the rat. Neurosci Res 36:327–334
Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N (1994) Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol 347:249–274
Li JL, Ding YQ, Li YQ, Li JS, Nomura S, Kaneko T, Mizuno N (1998) Immunocytochemical localization of mu-opioid receptor in primary afferent neurons containing substance P or calcitonin gene-related peptide. A light and electron microscope study in the rat. Brain Res 794:347–352
Li JL, Kaneko T, Shigemoto R, Mizuno N (1997) Distribution of trigeminohypothalamic and spinohypothalamic tract neurons displaying substance P receptor-like immunoreactivity in the rat. J Comp Neurol 378:508–521
Ding YQ, Takada M, Shigemoto R, Mizuno N (1995) Trigeminoparabrachial projection neurons showing substance P receptor-like immunoreactivity in the rat. Neurosci Res 23:415–418
Li JL, Ding YQ, Xiong KH, Li JS, Shigemoto R, Mizuno N (1998) Substance P receptor (NK1)-immunoreactive neurons projecting to the periaqueductal gray: distribution in the spinal trigeminal nucleus and the spinal cord of the rat. Neurosci Res 30:219–225
Li JL, Ding YQ, Shigemoto R, Mizuno N (1996) Distribution of trigeminothalamic and spinothalamic-tract neurons showing substance P receptor-like immunoreactivity in the rat. Brain Res 719:207–212
Fundytus ME (2001) Glutamate receptors and nociception: implications for the drug treatment of pain. CNS Drugs 15:29–58
Kaneko T, Fujiyama F (2002) Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res 42:243–250
Fremeau RT Jr, Voglmaier S, Seal RP, Edwards RH (2004) VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci 27:98–103
Ge SN, Li ZH, Tang J, Ma Y, Hioki H, Zhang T, Lu YC, Zhang FX, Mizuno N, Kaneko T, Liu YY, Lung MS, Gao GD, Li JL (2014) Differential expression of VGLUT1 or VGLUT2 in the trigeminothalamic or trigeminocerebellar projection neurons in the rat. Brain Struct Funct 219:211–229
Ge SN, Ma YF, Hioki H, Wei YY, Kaneko T, Mizuno N, Gao GD, Li JL (2010) Coexpression of VGLUT1 and VGLUT2 in trigeminothalamic projection neurons in the principal sensory trigeminal nucleus of the rat. J Comp Neurol 518:3149–3168
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic, San Diego
Nakamura K, Watakabe A, Hioki H, Fujiyama F, Tanaka Y, Yamamori T, Kaneko T (2007) Transiently increased colocalization of vesicular glutamate transporters 1 and 2 at single axon terminals during postnatal development of mouse neocortex: a quantitative analysis with correlation coefficient. Eur J Neurosci 26:3054–3067
Hioki H, Nakamura H, Ma YF, Konno M, Hayakawa T, Nakamura KC, Fujiyama F, Kaneko T (2010) Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Comp Neurol 518:668–686
Kuramoto E, Furuta T, Nakamura KC, Unzai T, Hioki H, Kaneko T (2009) Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cereb Cortex 19:2065–2077
Chamberlin NL, Saper CB (1995) Differential distribution of AMPA-selective glutamate receptor subunits in the parabrachial nucleus of the rat. Neuroscience 68:435–443
Parenti C, Arico G, Ronsisvalle G, Scoto GM (2012) Supraspinal injection of Substance P attenuates allodynia and hyperalgesia in a rat model of inflammatory pain. Peptides 34:412–418
Dubner R, Bennett GJ (1983) Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci 6:381–418
Craig AD, Dostrovsky JO (2001) Differential projections of thermoreceptive and nociceptive lamina I trigeminothalamic and spinothalamic neurons in the cat. J Neurophysiol 86:856–870
Dickenson AH, Le Bars D (1983) Diffuse noxious inhibitory controls (DNIC) involve trigeminothalamic and spinothalamic neurones in the rat. Exp Brain Res 49:174–180
Jergova S, Kolesar D, Cizkova D (2008) Expression of c-Fos in the parabrachial nucleus following peripheral nerve injury in rats. Eur J Pain 12:172–179
Bester H, Beggs S, Woolf CJ (2000) Changes in tactile stimuli-induced behavior and c-Fos expression in the superficial dorsal horn and in parabrachial nuclei after sciatic nerve crush. J Comp Neurol 428:45–61
Bester H, Matsumoto N, Besson JM, Bernard JF (1997) Further evidence for the involvement of the spinoparabrachial pathway in nociceptive processes: a c-Fos study in the rat. J Comp Neurol 383:439–458
Ding YQ, Takada M, Shigemoto R, Mizumo N (1995) Spinoparabrachial tract neurons showing substance P receptor-like immunoreactivity in the lumbar spinal cord of the rat. Brain Res 674:336–340
Hwang SJ, Burette A, Valtschanoff JG (2003) VR1-positive primary afferents contact NK1-positive spinoparabrachial neurons. J Comp Neurol 460:255–265
Benoliel R, Tanaka M, Caudle RM, Iadarola MJ (2000) Co-localization of N-methyl-d-aspartate receptors and substance P (neurokinin-1) receptors in rat spinal cord. Neurosci Lett 291:61–64
Okano K, Kuraishi Y, Satoh M (1998) Involvement of spinal substance P and excitatory amino acids in inflammatory hyperalgesia in rats. Jpn J Pharmacol 76:15–22
Yang K, Wang GD, Li YQ, Shi JW, Zhao ZQ (1998) Co-existence of glutamate and substance P in electrophysiologically identified dorsal root ganglion neurons of rats. Sheng Li Xue Bao 50:453–459
Battaglia G, Rustioni A (1988) Coexistence of glutamate and substance P in dorsal root ganglion neurons of the rat and monkey. J Comp Neurol 277:302–312
Zhang L, Hammond DL (2009) Substance P enhances excitatory synaptic transmission on spinally projecting neurons in the rostral ventromedial medulla after inflammatory injury. J Neurophysiol 102:1139–1151
Koganemaru M, Takasaki M, Nishimori T (2000) Simultaneous activation of N-methyl-d-aspartate and neurokinin-1 receptors modulates c-Fos and Zif/268 expression in the rat trigeminal nucleus caudalis. Neuroscience 98:317–323
Magnusson KR, Larson AA, Madl JE, Altschuler RA, Beitz AJ (1986) Co-localization of fixative-modified glutamate and glutaminase in neurons of the spinal trigeminal nucleus of the rat: an immunohistochemical and immunoradiochemical analysis. J Comp Neurol 247:477–490
Polgar E, Antal M (1995) The colocalization of parvalbumin and calbindin-D28k with GABA in the subnucleus caudalis of the rat spinal trigeminal nucleus. Exp Brain Res 103:402–408
Esteves FO, McWilliam PN, Batten TF (2000) Nitric oxide producing neurones in the rat medulla oblongata that project to nucleus tractus solitarii. J Chem Neuroanat 20:185–197
Pang YW, Ge SN, Nakamura KC, Li JL, Xiong KH, Kaneko T, Mizuno N (2009) Axon terminals expressing vesicular glutamate transporter VGLUT1 or VGLUT2 within the trigeminal motor nucleus of the rat: origins and distribution patterns. J Comp Neurol 512:595–612
Blomqvist A, Ericson AC, Craig AD, Broman J (1996) Evidence for glutamate as a neurotransmitter in spinothalamic tract terminals in the posterior region of owl monkeys. Exp Brain Res 108:33–44
Ghanbari A, Asgari AR, Kaka GR, Falahatpishe HR, Naderi A, Jorjani M (2014) In vivo microdialysis of glutamate in ventroposterolateral nucleus of thalamus following electrolytic lesion of spinothalamic tract in rats. Exp Brain Res 232:415–421
Cameron D, Polgar E, Gutierrez-Mecinas M, Gomez-Lima M, Watanabe M, Todd AJ (2015) The organisation of spinoparabrachial neurons in the mouse. Pain 156:2061–2071
Fremeau RT Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH (2004) Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science 304:1815–1819
Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, Buist A, Cik M, van der Spek P, Kass S, Meert T, D’Hooge R, Rosenmund C, Hampson RM (2006) Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J Neurosci 26:12055–12066
Leo S, Moechars D, Callaerts-Vegh Z, D’Hooge R, Meert T (2009) Impairment of VGLUT2 but not VGLUT1 signaling reduces neuropathy-induced hypersensitivity. Eur J Pain 13:1008–1017
Scherrer G, Low SA, Wang X, Zhang J, Yamanaka H, Urban R, Solorzano C, Harper B, Hnasko TS, Edwards RH, Basbaum AI (2010) VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc Natl Acad Sci USA 107:22296–22301
Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q (2010) VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron 68:543–556
Lagerstrom MC, Rogoz K, Abrahamsen B, Lind AL, Olund C, Smith C, Mendez JA, Wallen-Mackenzie A, Wood JN, Kullander K (2011) A sensory subpopulation depends on vesicular glutamate transporter 2 for mechanical pain, and together with substance P, inflammatory pain. Proc Natl Acad Sci USA 108:5789–5794
Rogoz K, Lagerstrom MC, Dufour S, Kullander K (2012) VGLUT2-dependent glutamatergic transmission in primary afferents is required for intact nociception in both acute and persistent pain modalities. Pain 153:1525–1536
Lin LH, Nitschke Dragon D, Talman WT (2012) Collateral damage and compensatory changes after injection of a toxin targeting neurons with the neurokinin-1 receptor in the nucleus tractus solitarii of rat. J Chem Neuroanat 43:141–148
Alden M, Besson JM, Bernard JF (1994) Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: a PHA-L study in the rat. J Comp Neurol 341:289–314
Saper CB (1995) The spinoparabrachial pathway: shedding new light on an old path. J Comp Neurol 353:477–479
Benarroch EE (2001) Pain-autonomic interactions: a selective review. Clin Auton Res 11:343–349
Bourgeais L, Gauriau C, Bernard JF (2001) Projections from the nociceptive area of the central nucleus of the amygdala to the forebrain: a PHA-L study in the rat. Eur J Neurosci 14:229–255
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 81571074).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xu Li and Shun-Nan Ge have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2016_2080_MOESM1_ESM.tif
Supplementary material 1. Supplement Fig.1 Microphotographs of sections through the middle level of a FG injection into the left side of thalamus site involving VPL and VPM (A,B), and CTB injection into the right side of PBN (C,D) in R2(A,C) and R5(B,D). Scale bar = 400 µm in A,B, 500 µm in C,D. (TIF 4447 KB)
Rights and permissions
About this article
Cite this article
Li, X., Ge, SN., Li, Y. et al. Neurokinin-1 Receptor-Immunopositive Neurons in the Medullary Dorsal Horn Provide Collateral Axons to both the Thalamus and Parabrachial Nucleus in Rats. Neurochem Res 42, 375–388 (2017). https://doi.org/10.1007/s11064-016-2080-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2080-0