Abstract
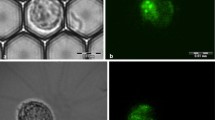
To investigate the cellular accumulation and intracellular localization of dimercaptosuccinate-coated iron oxide nanoparticles (D-IONPs) in oligodendroglial cells, we have synthesized IONPs that contain the fluorescent dye BODIPY (BP) in their coat (BP-D-IONPs) and have investigated the potential effects of the absence or presence of this dye on the particle uptake by oligodendroglial OLN-93 cells. Fluorescent BP-D-IONPs and non-fluorescent D-IONPs had similar hydrodynamic diameters and ζ-potentials of around 60 nm and −58 mV, respectively, and showed identical colloidal stability in physiological media with increasing particle size and positivation of the ζ-potential in presence of serum. After exposure of oligodendroglial OLN-93 cells to BP-D-IONPs or D-IONPs in the absence of serum, the specific cellular iron content increased strongly to around 1,800 nmol/mg. This strong iron accumulation was lowered for both types of IONPs by around 50 % on exposure of the cells at 4 °C and by around 90 % on incubation in presence of 10 % serum. The accumulation of both D-IONPs and BP-D-IONPs in the absence of serum was not affected by endocytosis inhibitors, whereas in the presence of serum inhibitors of clathrin-dependent endocytosis lowered the particle accumulation by around 50 %. These data demonstrate that oligodendroglial cells efficiently accumulate IONPs by an endocytotic process which is strongly affected by the temperature and the presence of serum and that BP-D-IONPs are a reliable tool to monitor by fluorescence microscopy the uptake and cellular fate of D-IONPs.






Similar content being viewed by others
References
Akbarzadeh A, Samiei M, Davaran S (2012) Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett 7:144
Mahmoudi M, Stroeve P, Milani A, Arbab A (2011) Superparamagnetic iron oxide nanoparticles—synthesis, surface engineering, cytotoxicity and biomedical applications. Nova Science Publishers Inc, New York
Nikiforov V, Filinova E (2009) Biomedical applications of magnetic nanoparticles. In: Gubin SP (ed) Magnetic nanoparticles. WILEY-VCH Verlag GmbH, Weinheim, pp 393–455
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26:3995–4021
Amstad E, Textor M, Reimhult E (2011) Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 3:2819–2843
Huang SH, Juang RS (2011) Biochemical and biomedical applications of multifunctional magnetic nanoparticles: a review. J Nanopart Res 13:4411–4430
Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M (2009) Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater 8:543–557
Kaewsaneha C, Opaprakasit P, Polpanich D, Smanmoo S, Tangboriboonrat P (2012) Immobilization of fluorescein isothiocyanate on magnetic polymeric nanoparticle using chitosan as spacer. J Colloid Interface Sci 377:145–152
Geppert M, Petters C, Thiel K, Dringen R (2013) Presence of serum alters properties of iron oxide nanoparticles and lowers their accumulation by cultured brain astrocytes. J Nanopart Res 15:1349
Petri-Fink A, Steitz B, Finka A, Salaklang J, Hofmann H (2008) Effect of cell media on polymer coated superparamagnetic iron oxide nanoparticles (SPIONs): colloidal stability, cytotoxicity, and cellular uptake studies. Eur J Pharm Biopharm 68:129–137
Wiogo HT, Lim M, Bulmus V, Yun J, Amal R (2011) Stabilization of magnetic iron oxide nanoparticles in biological media by fetal bovine serum (FBS). Langmuir 27:843–850
Fang C, Bhattarai N, Sun C, Zhang M (2009) Functionalized nanoparticles with long-term stability in biological media. Small 5:1637–1641
Xiong F, Zhu ZY, Xiong C, Hua XQ, Shan XH, Zhang Y, Gu N (2012) Preparation, characterization of 2-deoxy-d-glucose functionalized dimercaptosuccinic acid-coated maghemite nanoparticles for targeting tumor cells. Pharm Res 29:1087–1097
Zhu XM, Wang YX, Leung KC, Lee SF, Zhao F, Wang DW, Lai JM, Wan C, Cheng CH, Ahuja AT (2012) Enhanced cellular uptake of aminosilane-coated superparamagnetic iron oxide nanoparticles in mammalian cell lines. Int J Nanomed 7:953–964
Hohnholt M, Geppert M, Dringen R (2010) Effects of iron chelators, iron salts, and iron oxide nanoparticles on the proliferation and the iron content of oligodendroglial OLN-93 cells. Neurochem Res 35:1259–1268
Hohnholt MC, Dringen R (2011) Iron-dependent formation of reactive oxygen species and glutathione depletion after accumulation of magnetic iron oxide nanoparticles by oligodendroglial cells. J Nanopart Res 13:6761–6774
Hohnholt MC, Geppert M, Dringen R (2011) Treatment with iron oxide nanoparticles induces ferritin synthesis but not oxidative stress in oligodendroglial cells. Acta Biomater 7:3946–3954
Richter-Landsberg C, Heinrich M (1996) OLN-93: a new permanent oligodendroglia cell line derived from primary rat brain glial cultures. J Neurosci Res 45:161–173
Tulpule K, Schmidt MM, Boecker K, Goldbaum O, Richter-Landsberg C, Dringen R (2012) Formaldehyde induces rapid glutathione export from viable oligodendroglial OLN-93 cells. Neurochem Int 61:1302–1313
Auffan M, Decome L, Rose J, Orsiere T, De Meo M, Briois V, Chaneac C, Olivi L, Berge-Lefranc JL, Botta A, Wiesner MR, Bottero JY (2006) In vitro interactions between DMSA-coated maghemite nanoparticles and human fibroblasts: a physicochemical and cyto-genotoxical study. Environ Sci Technol 40:4367–4373
Fauconnier N, Pons JN, Roger J, Bee A (1997) Thiolation of maghemite nanoparticles by dimercaptosuccinic acid. J Colloid Interface Sci 194:427–433
Geppert M, Hohnholt MC, Nurnberger S, Dringen R (2012) Ferritin up-regulation and transient ROS production in cultured brain astrocytes after loading with iron oxide nanoparticles. Acta Biomater 8:3832–3839
Geppert M, Hohnholt MC, Thiel K, Nurnberger S, Grunwald I, Rezwan K, Dringen R (2011) Uptake of dimercaptosuccinate-coated magnetic iron oxide nanoparticles by cultured brain astrocytes. Nanotechnology 22:145101
Busch W, Bastian S, Trahorsch U, Iwe M, Kuhnel D, Meissner T, Springer A, Gelinsky M, Richter V, Ikonomidou C, Potthoff A, Lehmann I, Schirmer K (2011) Internalisation of engineered nanoparticles into mammalian cells in vitro: influence of cell type and particle properties. J Nanopart Res 13:293–310
Luther EM, Petters C, Bulcke F, Kaltz A, Thiel K, Bickmeyer U, Dringen R (2013) Endocytotic uptake of iron oxide nanoparticles by cultured brain microglial cells. Acta Biomater 9:8454–8465
Geppert M, Hohnholt M, Gaetjen L, Grunwald I, Baumer M, Dringen R (2009) Accumulation of iron oxide nanoparticles by cultured brain astrocytes. J Biomed Nanotechnol 5:285–293
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lamkowsky MC, Geppert M, Schmidt MM, Dringen R (2012) Magnetic field-induced acceleration of the accumulation of magnetic iron oxide nanoparticles by cultured brain astrocytes. J Biomed Mat Res A 100A:323–334
Hohnholt MC, Geppert M, Luther EM, Petters C, Bulcke F, Dringen R (2013) Handling of iron oxide and silver nanoparticles by astrocytes. Neurochem Res 38:227–239
Valois CR, Braz JM, Nunes ES, Vinolo MA, Lima EC, Curi R, Kuebler WM, Azevedo RB (2010) The effect of DMSA-functionalized magnetic nanoparticles on transendothelial migration of monocytes in the murine lung via a beta2 integrin-dependent pathway. Biomaterials 31:366–374
Mahmoudi M, Lynch I, Ejtehadi MR, Monopoli MP, Bombelli FB, Laurent S (2011) Protein-nanoparticle interactions: opportunities and challenges. Chem Rev 111:5610–5637
Tenzer S, Docter D, Rosfa S, Wlodarski A, Kuharev J, Rekik A, Knauer SK, Bantz C, Nawroth T, Bier C, Sirirattanapan J, Mann W, Treuel L, Zellner R, Maskos M, Schild H, Stauber RH (2011) Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: a comprehensive quantitative proteomic analysis. ACS Nano 5:7155–7167
Kim JS, Yoon TJ, Yu KN, Noh MS, Woo M, Kim BG, Lee KH, Sohn BH, Park SB, Lee JK, Cho MH (2006) Cellular uptake of magnetic nanoparticle is mediated through energy-dependent endocytosis in A549 cells. J Vet Sci 7:321–326
Smith PJ, Giroud M, Wiggins HL, Gower F, Thorley JA, Stolpe B, Mazzolini J, Dyson RJ, Rappoport JZ (2012) Cellular entry of nanoparticles via serum sensitive clathrin-mediated endocytosis, and plasma membrane permeabilization. Int J Nanomed 7:2045–2055
Pinkernelle J, Calatayud P, Goya GF, Fansa H, Keilhoff G (2012) Magnetic nanoparticles in primary neural cell cultures are mainly taken up by microglia. BMC Neurosci 13:32
Jenkins SI, Pickard MR, Furness DN, Yiu HH, Chari DM (2013) Differences in magnetic particle uptake by CNS neuroglial subclasses: implications for neural tissue engineering. Nanomedicine (Lond) 8:951–968
Ivanov AI (2008) Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol Biol 440:15–33
Iversen TG, Skotland T, Sandvig K (2011) Endocytosis and intracellular transport of nanoparticles: present knowledge and need for future studies. Nano Today 6:176–185
Butoescu N, Seemayer CA, Foti M, Jordan O, Doelker E (2009) Dexamethasone-containing PLGA superparamagnetic microparticles as carriers for the local treatment of arthritis. Biomaterials 30:1772–1780
Le PU, Guay G, Altschuler Y, Nabi IR (2002) Caveolin-1 is a negative regulator of caveolae-mediated endocytosis to the endoplasmic reticulum. J Biol Chem 277:3371–3379
Wang LH, Rothberg KG, Anderson RG (1993) Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol 123:1107–1117
Banbury DN, Oakley JD, Sessions RB, Banting G (2003) Tyrphostin A23 inhibits internalization of the transferrin receptor by perturbing the interaction between tyrosine motifs and the medium chain subunit of the AP-2 adaptor complex. J Biol Chem 278:12022–12028
Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T (2006) Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10:839–850
Iversen TG, Frerker N, Sandvig K (2012) Uptake of ricinB-quantum dot nanoparticles by a macropinocytosis-like mechanism. J Nanobiotechnol 10:33
Loubery S, Wilhelm C, Hurbain I, Neveu S, Louvard D, Coudrier E (2008) Different microtubule motors move early and late endocytic compartments. Traffic 9:492–509
Mejias R, Perez-Yague S, Roca AG, Perez N, Villanueva A, Canete M, Manes S, Ruiz-Cabello J, Benito M, Labarta A, Batlle X, Veintemillas-Verdaguer S, Morales MP, Barber DF, Serna CJ (2010) Liver and brain imaging through dimercaptosuccinic acid-coated iron oxide nanoparticles. Nanomedicine (Lond) 5:397–408
Acknowledgments
The authors like to thank Christiane Richter-Landsberg (University of Oldenburg) for kindly supplying us with OLN-93 cells and Michaela C. Hohnholt (University of Bremen) for critically reading the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petters, C., Bulcke, F., Thiel, K. et al. Uptake of Fluorescent Iron Oxide Nanoparticles by Oligodendroglial OLN-93 Cells. Neurochem Res 39, 372–383 (2014). https://doi.org/10.1007/s11064-013-1234-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1234-6