Abstract
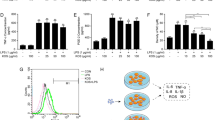
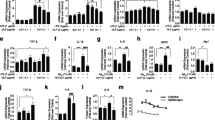
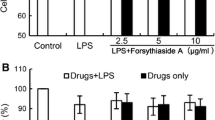
The major neurodegenerative diseases are characterized by increasing of activated-microglial cells and inflammatory cytokines in the central nervous system. Carrageenan extracted from red algae is a kind of polysaccharide with sulfate groups. The oligosaccharides were obtained from carrageenan by enzymatic degradation. To detect the immunomodulatory activity of κ-carrageenan oligosaccharides (KOS) on microglial cells and the relationship to the sulfate group content, the desulfated derivatives of KOS (DSK) were obtained by dimethyl sulfoxide–methanol–pyridine method. KOS was labeled with fluorescein isothiocyanate. The effect of KOS and DSK on lipopolysaccharide (LPS)-activated microglial cells was detected. Hematoxylin–eosin staining and flow cytometric were used to detect the cell viability. The “scratch” migration assay, ornithine analysis and RT-PCR were used to determine the cell migration, arginase and TNF-α released by microglial cells, respectively. The effect of LPS and KOS on microglial cells was determined by flow cytometry and laser scanning confocal microscopy. The results showed that KOS and DSK could inhibit the cell viability, arginase and TNF-α released by LPS-activated microglia cell with concentration dependent manner. But the effect of DSK was weaker than that of KOS. KOS aggregated on the cell surface firstly, and then they enter into the cell to the nucleus, spread over the entire cell finally. But the exist of LPS could prevent the entrance of KOS. It could be concluded that KOS could protect microglial cells from being activated by LPS, and its inhibition function had relationship to the sulfate group content of KOS, while there were competition between LPS and KOS.








Similar content being viewed by others
Abbreviations
- KOS:
-
κ-Carrageenan
- LPS:
-
Lipopolysaccharide
- DSK:
-
Desulfated derivatives of κ-carrageenan oligosaccharides
- IMDM:
-
Iscove’s modified Dulbecco’s medium
- FBS:
-
Fetal bovine serum
- DMSO:
-
Dimethyl sulfoxide
- MWCO:
-
Molecular weight cut off
- PI:
-
Propidium iodide
- FITC:
-
Fluorescein isothiocyanate
- FITC-KOS:
-
Fluorescently labeled κ-carrageenan oligosaccharides
- OD:
-
Optical density
References
Aloisi F (2001) Immune function of microglia. Glia 36:165–179
Amano F, Akamatsu Y (1991) A lipopolysaccharide (LPS)-resistant mutant isolated from a macrophagelike cell line, J774.1, exhibits an altered activated-macrophage in response to LPS. Infect Immun 59:2166–2174
Barahona T, Chandía NP, Encinas MV, Matsuhiroa B, Zúñiga EA (2011) Antioxidant capacity of sulfated polysaccharides from seaweeds. A kinetic approach. Food Hydrocoll 25(3):529–535
Benveniste EN, Nguyen VT, O’Keefe GM (2001) Immunological aspects of microglia: relevance to Alzheimer’s disease. Neurochem Int 39(5–6):381–391
Bouarab K, Potin P, Correa J, Kloareg B (1999) Sulfated oligosaccharides mediate the interaction between a marine red alga and its green algal pathogenic endophyte. Plant Cell 11(9):1635–1650
Cao L, Su Z, Zhou Q, Lv BL, Liu XJ, Jiao L, Li ZH, Zhu YL, Huang ZH, Huang AJ, He C (2006) Glial cell line-derived neurotrophic factor promotes olfactory ensheathing cells migration. Glia 54(6):536–544
Carluccim MJ, Pujolc CA, Cianciam M, Noseda MD, Matulewicz MC, Damonte EB, Cerezo AS (1997) Antiherpetic and anticoagulant properties of carrageenans from the red seaweed Gigartina skottsbergii and their cyclized derivatives: correlation between structure and biological activity. Int J Biol Macromol 20:97–105
Colton CA (2013) Microglial-neuronal interactions during neurodegenerative diseases. J Neuroimmune Pharmacol 8(1):4–6
Davis DS, Carson MJ (2013) An introduction to CNS-resident microglia: definitions, assays, and functional roles in health and disease. Neural-immune interactions in brain function and alcohol related disorders. Springer, US, pp 3–29
Dodgson KS (1961) Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem J 78:312–319
Dun JX, Yao ZA, Wu HG, Wang FF (2010) Isolation, characterization of kappa-carrageenase-producing strain and preliminary analysis of hydrolysis products. Food Sci Technol 35(4):11–16
Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P, Virgilio FD (1996) Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J Immunol 156:1531–1539
Gless CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140(6):918–934
Gómez-Nicola D, Fransen NL, Suzzi S et al (2013) Regulation of microglial proliferation during chronic neurodegeneration. J Neurosci 33(6):2481–2493
Gomori G (1939) A differential stain for cell types in the pancreatic islets. Am J Pathol 15(4):497–499
Gu H (2013) Using induced pluripotent stem cells to model neurodegenerative diseases. J Anc Dis Prev Rem 1(1):2
Guven KC, Ozsoy Y, Ulutin ON (2009) Anticoagulant, fibrinolytic and antiaggregant activity of carrageenans and alginic acid. Bot Mar 34:429–432
Hedley DW, Friedlander ML, Taylor IW, Rugg CA, Musgrove EA (1983) Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem 31(11):1333–1335
Hickman SE, Allison EK, Khoury JE (2008) Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci 28(33):8354–8360
Hu X, Jiang XL, Aubree E, Boulenguer P, Critchley AT (2006) Preparation and in vivo. Antitumor activity of κ-carrageenan oligosaccharides. Pharm Biol 44(9):646–650
Kawai Y (1969) Modified methods to determine sulfate in polysaccharide. Anal Chem 32:314–321
Klarzynsk O, Descamps V, Plesse B, Yvin JC, Kloareg B, Fritig B (2003) Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Am Phytopathol Soc 16(2):115–122
Marinova-Mutafchieva L, Sadeghian M, Broom L, Davis JB, Medhurst AD, Dexter DT (2009) Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: a time course study in a 6-hydroxydopamine model of Parkinson’s disease. J Neurochem 110(3):966–975
Meintanis S, Thomaidou D, Jessen KR et al (2001) The neuron–glia signal β neuregulin promotes Schwann cell motility via the MAPK pathway. Glia 34(1):39–51
Miller IJ, Blrnt JW (1998) Desulfation of algal glactans. Carbohydr Res 309(1):39–43
Pauleau AL, Rutschman R, Lang R, Pernis A, Watowich SS, Murray PJ (2004) Enhancer-mediated control of macrophage-specific arginase I expression. J Immunol 172(12):7565–7573
Perry VH, Teeling J (2013) Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Springer, Berlin, pp 1–12
Pool CR (1969) Hematoxylin–eosin staining of OsO4-fixed epon-embedded tissue; prestaining oxidation by acidified H2O2. Biotech Histochem 44(2):75–79
Rees DA (1970) Structure, conformation, and mechanism in the formation of polysaccharide gels and networks. Adv Carbohydr Chem Biochem 24:267–332
Riccardi C, Nicoletti I (2006) Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc 1:1458–1461
Rochas C, Lahaye M, Yaphe W (1986) Sulfate content of carrageenan and Agar determined by infrared spectroscopy. Bot Mar 29(4):335–340
Singh S, Metz I, Amor S, et al (2013) Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol 125(4):595–608
Streit WJ (2002) Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 40(2):133-139
Sun T, Tao HN, Zhou DX (2009) Study on antioxidant activity of κ-carrageenan oligosaccharides by oxidation degradation and acidic degradation. Sci Technol Food Ind 30:111–112
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2013(126):663–676
Vermes I, Haanen C, Steffens-Nakken H, Reutellingsperger C (1995) A novel assay for apoptosis flow cytometric detection of phosphatidylserine early apoptotic cells using fluorescein labeled expression on Annexin V. J Immunol Methods 184(1):39–51
Wang F, Yao Z, Wu HG, Zhang SX, Zhu NN, Gai X (2011) Antibacterial activities of kappa-carrageenan oligosaccharides. Appl Mech Mater 108:194–199
Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R (2003) Activation of microglia by human neuromelanin is NF-κB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson’s disease. J Fed Am Soc Exp Biol 17(3):500–502
Xiong X, White RE, Xu L et al (2013) Mitigation of murine focal cerebral ischemia by the hypocretin/orexin system is associated with reduced inflammation. Stroke 44(3):764–770
Xu L, Yao Z, Wu HG, Wang FF, Zhang SX (2012) The immune regulation of κ-carrageenan oligosaccharide and its desulfated derivatives on LPS-activated microglial cells. Neurochem Int 61(5):689–696
Yao L, Kan EM, Lu J et al (2013) Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J Neuroinflammation 10(1):23
Yuan H, Song JM, Li XG, Li N, Dai JC (2006) Immunomodulation and antitumor activity of κ-carrageenan oligosaccharides. Cancer Lett 243(2):228–234
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81070973 and 81373429).
Author information
Authors and Affiliations
Corresponding author
Additional information
Zi-ang Yao and Ling Xu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yao, Za., Xu, L. & Wu, Hg. Immunomodulatory Function of κ-Carrageenan Oligosaccharides Acting on LPS-Activated Microglial Cells. Neurochem Res 39, 333–343 (2014). https://doi.org/10.1007/s11064-013-1228-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1228-4