Abstract
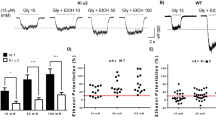
γ-Aminobutyric acid type A receptors (GABAA-Rs) are considered to be the primary molecular targets of injectable anesthetics such as propofol, etomidate and the neurosteriod, alphaxalone. A number of studies have sought to understand the specific GABAA-R subtypes involved in the mechanism of action of these three drugs. Here, we investigated the role of α4-subunit containing GABAA-Rs in the neurobehavioral responses to these drugs. Drug responses in α4 subunit knockout (KO) mice were compared to wild type (WT) littermate controls. While etomidate and propofol are currently used as injectable anesthetics, alphaxalone belongs to the class of neurosteroid drugs having anesthetic effects. Low dose effects of etomidate and alphaxalone were studied using an open field assay. The moderate and high dose effects of all three anesthetics were measured using the rotarod and loss of righting reflex assays, respectively. The locomotor stimulatory effect of alphaxalone was reduced significantly in α4 KO mice compared to WT controls. Neither the low dose sedating effect of etomidate, nor the moderate/high dose effect of any of the drugs differed between genotypes. These results suggest that α4 subunit-containing GABAA-Rs are required for the low dose, locomotor stimulatory effect of alphaxalone but are not required for the sedating effect of etomidate or the moderate/high dose effects of etomidate, propofol or alphaxalone on motor ataxia and loss of righting reflex.



Similar content being viewed by others
References
Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ (1998) International Union of pharmacology. XV. Subtypes of γ-aminobutyric acid A receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 50:291–313
Tretter V, Ehya N, Fuchs K, Sieghart W (1997) Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci 17:2728–2737
Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Mohler H (1992) Five subtypes of type A γ-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci USA 89:6726–6730
Liao M, Sonner JM, Husain SS, Miller KW, Jurd R, Rudolph U, Eger EI II (2005) R (+) etomidate and the photoactivable R (+) azietomidate have comparable anesthetic activity in wild-type mice and comparably decreased activity in mice with a N265M point mutation in the γ-aminobutyric acid receptor β3 subunit. Anesth Analg 101:131–135
Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U (2003) General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor β3 subunit. FASEB J 17:250–252
Reynolds DS, Rosahl TW, Cirone J, O’Meara GF, Haythornthwaite A, Newman RJ, Myers J, Sur C, Howell O, Rutter AR, Atack J, Macaulay AJ, Hadingham KL, Hutson PH, Belelli D, Lambert JJ, Dawson GR, McKernan R, Whiting PJ, Wafford KA (2003) Sedation and anesthesia mediated by distinct GABAA receptor isoforms. J Neurosci 23:8608–8617
Zeller A, Arras M, Lazaris A, Jurd R, Rudolph U (2005) Distinct molecular targets for the central respiratory and cardiac actions of the general anesthetics etomidate and propofol. FASEB J 19:1677–1679
Cirone J, Rosahl TW, Reynolds DS, Newman RJ, O’Meara GF, Hutson PH, Wafford KA (2004) γ-Aminobutyric acid type A receptor β2 subunit mediates the hypothermic effect of etomidate in mice. Anesthesiology 100:1438–1445
Quinlan JJ, Homanics GE, Firestone LL (1998) Anesthesia sensitivity in mice that lack the β3 subunit of the γ-aminobutyric acid type A receptor. Anesthesiology 88:775–780
Blednov YA, Jung S, Alva H, Wallace D, Rosahl T, Whiting PJ, Harris RA (2003) Deletion of the α1 or β2 subunit of GABAA receptors reduces actions of alcohol and other drugs. J Pharmacol Exp Ther 304:30–36
Ferguson C, Hardy SL, Werner DF, Hileman SM, Delorey TM, Homanics GE (2007) New insight into the role of the β3 subunit of the GABAA-R in development, behavior, body weight regulation, and anesthesia revealed by conditional gene knockout. BMC Neurosci 8:85
Mihalek RM, Banjeree PK, Korpi E, Quinlan JJ, Firestone LL, Mi Z-P, Lagenaur C, Tretter V, Sieghart W, Anagnostaras S, Sage JR, Fanselow M, Guidotti A, Spigelman I, Li A, DeLorey TM, Olsen RW, Homanics GE (1999) Attenuated sensitivity to neuroactive steroids in GABA type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA 96:12905–12910
Nusser Z, Sieghart W, Somogyi P (1998) Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 18:1693–1703
Wei W, Zhang N, Peng Z, Houser CR, Mody I (2003) Perisynaptic localization of delta subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci 23:10650–10661
Nusser Z, Mody I (2002) Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophys 87:2624–2628
Orser BA (2006) Extrasynaptic GABAA receptors are critical targets for sedative-hypnotic drugs. J Clin Sleep Med 2:S12–S18
Laurie DJ, Seeburg PH, Wisden W (1992) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci 12:1063–1076
Laurie DJ, Wisden W, Seeburg PH (1992) The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci 12:4151–4172
Wisden W, Laurie DJ, Monyer H, Seeburg PH (1992) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci 12:1040–1062
Maguire J, Mody I (2009) Steroid hormone fluctuations and GABAAR plasticity. Psychoneuroendocrinology 34(Suppl 1):S84–S90
Shen H, Smith SS (2009) Plasticity of the α4βδ GABAA receptor. Biochem Soc Trans 37:1378–1384
Maguire J, Ferando I, Simonsen C, Mody I (2009) Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci 29:9592–9601
Maguire J, Mody I (2008) GABAAR plasticity during pregnancy: relevance to postpartum depression. Neuron 59:207–213
Meera P, Olsen RW, Otis TS, Wallner M (2009) Etomidate, propofol and the neurosteroid THDOC increase the GABA efficacy of recombinant α4β3δ and α4β3 GABAA receptors expressed in HEK cells. Neuropharmacology 56:155–160
Li GD, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB (2006) Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci 26:11599–11605
Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE (2006) GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA 103:15230–15235
Rau V, Iyer SV, Oh I, Chandra D, Harrison N, Eger EI 2nd, Fanselow MS, Homanics GE, Sonner JM (2009) γ-Aminobutyric acid type A receptor α4 subunit knockout mice are resistant to the amnestic effect of isoflurane. Anesth Analg 109:1816–1822
Liang J, Suryanarayanan A, Chandra D, Homanics GE, Olsen RW, Spigelman I (2008) Functional consequences of GABAA receptor α4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol Clin Exp Res 32:19–26
Crawley JN (1985) Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev 9:37–44
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3–33
Stell BM, Brickley SG, Tang CY, Farrant M, Mody I (2003) Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci USA 100:14439–14444
Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I (2007) A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci 10:40–48
Kralic JE, Wheeler M, Renzi K, Ferguson C, O’Buckley TK, Grobin AC, Morrow AL, Homanics GE (2003) Deletion of GABAA receptor α1 subunit-containing receptors alters responses to ethanol and other anesthetics. J Pharmacol Exp Ther 305:600–607
Maguire JL, Stell BM, Rafizadeh M, Mody I (2005) Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 8:797–804
Cheng VY, Martin LJ, Elliott EM, Kim JH, Mount HT, Taverna FA, Roder JC, Macdonald JF, Bhambri A, Collinson N, Wafford KA, Orser BA (2006) α5 GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci 26:3713–3720
Siegwart R, Jurd R, Rudolph U (2002) Molecular determinants for the action of general anesthetics at recombinant α3β2γ2 γ-aminobutyric acid(A) receptors. J Neurochem 80:140–148
Siegwart R, Krahenbuhl K, Lambert S, Rudolph U (2003) Mutational analysis of molecular requirements for the actions of general anaesthetics at the γ-aminobutyric acidA receptor subtype, α1β2γ2. BMC Pharmacol 3:13
Jonsson Fagerlund M, Sjodin J, Krupp J, Dabrowski MA (2010) Reduced effect of propofol at human α1β2(N289M)γ2 and α2β3(N290M)γ2 mutant GABAA receptors. Br J Anaesth 104:472–481
Wallner M, Hanchar HJ, Olsen RW (2006) Low-dose alcohol actions on α4β3δ GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc Natl Acad Sci USA 103:8540–8545
Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW (2006) Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to α4/6β3δ GABAA receptors. Proc Natl Acad Sci USA 103:8546–8551
Cushman JD, Moore MD, Jacobs NS, Olsen RW, Fanselow MS (2011) Behavioral pharmacogenetic analysis on the role of the α4 GABAA receptor subunit in the ethanol-mediated impairment of hippocampus-dependent contextual learning. Alcohol Clin Exp Res 35:1948–1959
Chandra D, Werner DF, Liang J, Suryanarayanan A, Harrison NL, Spigelman I, Olsen RW, Homanics GE (2008) Normal acute behavioral responses to moderate/high dose ethanol in GABAA receptor α4 subunit knockout mice. Alcohol Clin Exp Res 32:10–18
Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR (2002) GABAA receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J Comp Neurol 446:179–197
Suryanarayanan A, Liang J, Meyer EM, Lindemeyer AK, Chandra D, Homanics GE, Sieghart W, Olsen RW, Spigelman I (2011) Subunit compensation and plasticity of synaptic GABAA receptors induced by ethanol in α4 subunit knockout mice. Front Neurosci 5:110
Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I (2006) Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci 26:1749–1758
Acknowledgments
The authors would like to thank Erik Bennet and Carolyn Ferguson for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iyer, S.V., Chandra, D. & Homanics, G.E. GABAA-R α4 Subunits are Required for the Low Dose Locomotor Stimulatory Effect of Alphaxalone, But Not for Several Other Behavioral Responses to Alphaxalone, Etomidate or Propofol. Neurochem Res 39, 1048–1056 (2014). https://doi.org/10.1007/s11064-013-1148-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1148-3