Abstract
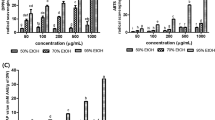
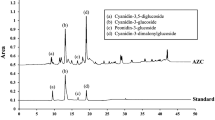
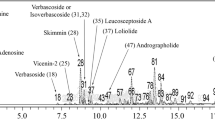
Oxidative stress mediates the cell damage in several ailments including neurodegenerative conditions. Ocimum sanctum is widely used in Indian ayurvedic medications to cure various ailments. The present study was carried out to investigate the antioxidant activity and neuroprotective effects of hydroalcoholic extract of O. sanctum (OSE) on hydrogen peroxide (H2O2)-induced oxidative challenge in SH-SY5Y human neuronal cells. The extract exhibited strong antioxidant activity against DPPH, 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) radical and hydroxyl radicals with IC50 values of 395 ± 16.2, 241 ± 11.5 and 188.6 ± 12.2 μg/ml respectively, which could be due to high amount of polyphenols and flavonoids. The observed data demonstrates 41.5 % cell survival with 100 μM H2O2 challenge for 24 h, which was restored to 73 % by pre-treatment with OSE for 2 h. It also decreased the lactate dehydrogenase leakage and preserved the cellular morphology. Similarly OSE inhibited lipid peroxidation, DNA damage, reactive oxygen species generation and depolarization of mitochondrial membrane. The extract restored superoxide dismutase and catalase enzyme/protein levels and further downregulated HSP-70 over-expression. These findings suggest that OSE ameliorates H2O2 induced neuronal damage via its antioxidant defence mechanism and might be used to treat oxidative stress mediated neuronal disorders.






Similar content being viewed by others
References
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82:291–295
Halliwell B (2012) Free radicals and antioxidants: updating a personal view. Nutr Rev 70:257–265
Jimenez-Del-Rio M, Velez-Pardo C (2012) The bad, the good, and the ugly about oxidative stress. Oxid Med Cell Longev 2012:1–13
Gupta SK, Prakash J, Srivastava S (2002) Validation of traditional claim of Tulsi, Ocimum sanctum Linn. as a medicinal plant. Indian J Exp Biol 40:765–773
Dutta D, Devi SS, Krishnamurthi K et al (2007) Modulatory effect of Ocimum sanctum leaf extract (Tulsi) on human lymphocytes against genotoxicants. Biomed Environ Sci 20:226–234
Ahmad A, Khan MM, Raza SS et al (2012) Ocimum sanctum attenuates oxidative damage and neurological deficits following focal cerebral ischemia/reperfusion injury in rats. Neurol Sci 33:1239–1247
Dharmani P, Kuchibhotla VK, Maurya R et al (2004) Evaluation of anti-ulcerogenic and ulcer-healing properties of Ocimum sanctum Linn. J Ethnopharmacol 93:197–206
Shah K, Verma RJ (2012) Protection against butyl p-hydroxybenzoic acid induced oxidative stress by Ocimum sanctum extract in mice liver. Acta Pol Pharm 69:865–870
Singh S, Majumdar DK (1999) Evaluation of the gastric antiulcer activity of fixed oil of Ocimum sanctum (Holy Basil). J Ethnopharmacol 65:13–19
Mediratta PK, Sharma KK, Singh S (2002) Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. J Ethnopharmacol 80:15–20
Joshi H, Parle M (2006) Evaluation of nootropic potential of Ocimum sanctum Linn. in mice. Indian J Exp Biol 44:133–136
Giridharan VV, Thandavarayan RA, Mani V et al (2011) Ocimum sanctum Linn. leaf extracts inhibit acetylcholinesterase and improve cognition in rats with experimentally induced dementia. J Med Food 14:912–919
Quéré L, Wenger T, Schramm HJ (1996) Triterpenes as potential dimerization inhibitors of HIV-1 protease. Biochem Biophys Res Commun 227:484–488
Chattopadhyay D, Arunachalam G, Mandal AB et al (2002) Antimicrobial and anti-inflammatory activity of folklore: Mallotus peltatus leaf extract. J Ethnopharmacol 82:229–237
Ovesná Z, Kozics K, Slamenová D (2006) Protective effects of ursolic acid and oleanolic acid in leukemic cells. Mutat Res 600:131–137
Venu Prasad MP, Khanum F (2012) Antifatigue activity of ethanolic extract of Ocimum sanctum in rats. Res J Med Plant 6:37–46
Xie HR, Hu LS, Li GY (2010) SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin Med J 123:1086–1092
Park SE, Kim S, Sapkota K et al (2010) Neuroprotective effect of Rosmarinus officinalis extract on human dopaminergic cell line, SH-SY5Y. Cell Mol Neurobiol 30:759–767
Kujala TS, Loponen JM, Klika KD et al (2000) Phenolic and betacyanins in red beetroot (Beta vulgaris) root: distribution and effects of cold storage on the content of total phenolics and three individual compounds. J Agric Food Chem 48:5338–5342
Delcour J, Varebeke DJ (1985) A new colorimetric assay for flavonoids in Pilsnerbeers. J Inst Brew 91:37–40
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Dinis TC, Maderia VM, Almeida LM (1994) Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 315:161–169
Kunchandy E, Rao MNA (1990) Oxygen radical scavenging activity of curcumin. Int J Pharm 58:237–240
Re R, Pellegrini N, Proteggente A et al (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 7:248–254
Cohen G, Dembiec D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34:30–38
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616
Singh NP, McCoy MT, Tice RR et al (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Qu L, Chen H, Liu X et al (2011) Neuroprotective properties of Spanish red wine and its isolated polyphenols on astrocytes. Food Chem 128:40–48
Martín S, González-Burgos E, Carretero ME et al (2011) Neuroprotective properties of Spanish red wine and its isolated polyphenols on astrocytes. Food Chem 128:40–48
Skaltsa H, Tzakou O, Singh M (1999) Note Polyphenols of Ocimum sanctum from Suriname. Pharm Biol 37:92–94
Kaul D, Shukla AR, Sikand K et al (2005) Effect of herbal polyphenols on atherogenic transcriptome. Mol Cell Biochem 278:177–184
Esmaeili MA, Sonboli A, Noushabadi MA (2010) Antioxidant and protective properties of six Tanacetum species against hydrogen peroxide-induced oxidative stress in K562 cell line: a comparative study. Food Chem 121:148–155
Steele ML, Truong J, Govindaraghavan S et al (2012) Cytoprotective properties of traditional Chinese medicinal herbal extracts in hydrogen peroxide challenged human U373 astroglia cells. Neurochem Int Sep 11. pii: S0197-0186(12)00286-0. doi:10.1016/j.neuint.2012.08.018
Choi BS, Sapkota K, Kim S et al (2010) Antioxidant activity and protective effects of Tripterygium regelii extract on hydrogen peroxide-induced injury in human dopaminergic cells, SH-SY5Y. Neurochem Res 35:1269–1280
Kumar KH, Khanum F (2013) Hydroalcoholic extract of Cyperus rotundus ameliorates H2O2-induced human neuronal cell damage via its anti-oxidative and anti-apoptotic machinery. Cell Mol Neurobiol 33:5–17
Nikam S, Nikam P, Ahaley SK et al (2009) Oxidative stress in parkinson’s disease. Indian J Clin Biochem 24:98–101
Mansouri MT, Farbood Y, Sameri MJ (2013) Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem 138:1028–1033
Ramesh B, Satakopan VN (2010) Antioxidant activities of hydroalcoholic extract of Ocimum sanctum against cadmium induced toxicity in rats. Indian J Clin Biochem 25:307–310
Ahmad A, Rasheed N, Gupta P et al (2012) Novel Ocimumoside A and B as anti-stress agents: modulation of brain monoamines and antioxidant systems in chronic unpredictable stress model in rats. Phytomedicine 19:639–647
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2’,7’-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Zhao L, Wang JL, Wang YR et al (2013) Apigenin attenuates copper-mediated β-amyloid neurotoxicity through antioxidation, mitochondrion protection and MAPK signal inactivation in an AD cell model. Brain Res 1492:33–35
Niki E (2009) Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med 47:469–484
Sanders LH, Timothy Greenamyre J (2013) Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic Biol Med pii: S0891-5849(13)00004-X. doi:10.1016/j.freeradbiomed.2013.01.003
Halder N, Joshi S, Nag TC et al (2009) Ocimum sanctum extracts attenuate hydrogen peroxide cytotoxic ultrastructural changes in human lens epithelial cells. Phytother Res 23:1734–1737
Kumar H, Lim HW, More SV et al (2012) The role of free radicals in the aging brain and Parkinson’s disease: convergence and parallelism. Int J Mol Sci 13:10478–10504
Baumgartner A, Kurzawa-Zegota M, Laubenthal J et al (2012) Comet-assay parameters as rapid biomarkers of exposure to dietary/environmental compounds an in vitro feasibility study on spermatozoa and lymphocytes. Mutat Res 743:25–35
Khanna A, Shukla P, Tabassum S (2011) Role of Ocimum sanctum as a genoprotective agent on chlorpyrifos-induced genotoxicity. Toxicol Int 18:9–13
Beere HM (2004) The stress of dying: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci 117:2641–2651
Hemanth Kumar K, Tamatam A, Pal A et al (2013) Neuroprotective effects of Cyperus rotundus on SIN-1 induced nitric oxide generation and protein nitration: ameliorative effect against apoptosis mediated neuronal cell damage. Neurotoxicology 34:150–159
Acknowledgments
The authors are highly thankful to Dr. HV Batra, Director, DFRL, Mysore for constant encouragement throughout the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venuprasad, M.P., Hemanth Kumar, K. & Khanum, F. Neuroprotective Effects of Hydroalcoholic Extract of Ocimum sanctum Against H2O2 Induced Neuronal Cell Damage in SH-SY5Y Cells via Its Antioxidative Defence Mechanism. Neurochem Res 38, 2190–2200 (2013). https://doi.org/10.1007/s11064-013-1128-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1128-7