Abstract
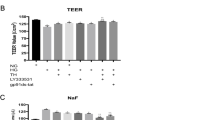
The blood–brain-barrier (BBB) is formed by different cell types, of which brain microvascular endothelial cells are major structural constituents. The goal of this study was to examine the effects of cooling on the permeability of the BBB with reference to tight junction formation of brain microendothelial cells. The sensorimotor cortex above the dura mater in adult male Wistar rats was focally cooled to a temperature of 5 °C for 1 h, then immunostaining for immunoglobulin G (IgG) was performed to evaluate the permeability of the BBB. Permeability produced by cooling was also evaluated in cultured murine brain endothelial cells (bEnd3) based on measurement of trans-epithelial electric resistance (TEER). Immunocytochemistry and Western blotting of proteins associated with tight junctions in bEnd3 were performed to determine protein distribution before and after cooling. After focal cooling of the rat brain cortex, diffuse immunostaining for IgG was observed primarily around the small vasculature and in the extracellular spaces of parenchyma of the cortex. In cultured bEnd3, TEER significantly decreased during cooling (15 °C) and recovered to normal levels after rewarming to 37 °C. Immunocytochemistry and Western blotting showed that claudin-5, a critical regulatory protein for tight junctions, was translocated from the membrane to the cytoplasm after cooling in cultured bEnd3 cells. These results suggest that focal brain cooling may open the BBB transiently through an effect on tight junctions of brain microendothelial cells, and that therapeutically this approach may allow control of BBB function and drug delivery through the BBB.




Similar content being viewed by others
References
Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnar Z, O’Donnell ME, Povlishock JT, Saunders NR, Sharp F, Stanimirovic D, Watts RJ, Drewes LR (2011) Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci 12(3):169–182. doi:10.1038/nrn2995
Takeshita Y, Ransohoff RM (2012) Inflammatory cell trafficking across the blood-brain barrier: chemokine regulation and in vitro models. Immunol Rev 248(1):228–239. doi:10.1111/j.1600-065X.2012.01127.x
Liu WY, Wang ZB, Zhang LC, Wei X, Li L (2012) Tight junction in blood-brain barrier: an overview of structure, regulation, and regulator substances. CNS Neurosci Ther 18(8):609–615. doi:10.1111/j.1755-5949.2012.00340.x
Fernandez-Lopez D, Faustino J, Daneman R, Zhou L, Lee SY, Derugin N, Wendland MF, Vexler ZS (2012) Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat. J Neurosci 32(28):9588–9600. doi:10.1523/JNEUROSCI.5977-11.2012
Esen F, Senturk E, Ozcan PE, Ahishali B, Arican N, Orhan N, Ekizoglu O, Kucuk M, Kaya M (2012) Intravenous immunoglobulins prevent the breakdown of the blood-brain barrier in experimentally induced sepsis. Crit Care Med 40(4):1214–1220. doi:10.1097/CCM.0b013e31823779ca
Koto T, Takubo K, Ishida S, Shinoda H, Inoue M, Tsubota K, Okada Y, Ikeda E (2007) Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol 170(4):1389–1397. doi:10.2353/ajpath.2007.060693
Chen G, Davies MA (2012) Emerging insights into the molecular biology of brain metastases. Biochem Pharmacol 83(3):305–314. doi:10.1016/j.bcp.2011.09.012
Helms HC, Waagepetersen HS, Nielsen CU, Brodin B (2010) Paracellular tightness and claudin-5 expression is increased in the BCEC/astrocyte blood-brain barrier model by increasing media buffer capacity during growth. AAPS J 12(4):759–770. doi:10.1208/s12248-010-9237-6
Cote J, Bovenzi V, Savard M, Dubuc C, Fortier A, Neugebauer W, Tremblay L, Muller-Esterl W, Tsanaclis AM, Lepage M, Fortin D, Gobeil F Jr (2012) Induction of selective blood-tumor barrier permeability and macromolecular transport by a biostable kinin B1 receptor agonist in a glioma rat model. PLoS ONE 7(5):e37485. doi:10.1371/journal.pone.0037485PONE-D-12-05042
Ramirez SH, Hasko J, Skuba A, Fan S, Dykstra H, McCormick R, Reichenbach N, Krizbai I, Mahadevan A, Zhang M, Tuma R, Son YJ, Persidsky Y (2012) Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J Neurosci 32(12):4004–4016. doi:10.1523/JNEUROSCI.4628-11.2012
Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O’Banion MK (2007) Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci 27(35):9301–9309. doi:10.1523/JNEUROSCI.1418-07.2007
Saligrama N, Noubade R, Case LK, del Rio R, Teuscher C (2012) Combinatorial roles for histamine H1–H2 and H3–H4 receptors in autoimmune inflammatory disease of the central nervous system. Eur J Immunol 42(6):1536–1546. doi:10.1002/eji.201141859
Park SJ, Lee JH, Kim HY, Choi YH, Park JS, Suh YH, Park SM, Joe EH, Jou I (2012) Astrocytes, but not microglia, rapidly sense H(2)O(2)via STAT6 phosphorylation, resulting in cyclooxygenase-2 expression and prostaglandin release. J Immunol 188(10):5132–5141. doi:10.4049/jimmunol.1101600
Pinzon-Daza ML, Garzon R, Couraud PO, Romero IA, Weksler B, Ghigo D, Bosia A, Riganti C (2012) The association of statins plus LDL receptor-targeted liposome-encapsulated doxorubicin increases the in vitro drug delivery across blood-brain barrier cells. Br J Pharmacol. doi:10.1111/j.1476-5381.2012.02103.x
Rempe R, Cramer S, Huwel S, Galla HJ (2011) Transport of Poly(n-butylcyano-acrylate) nanoparticles across the blood-brain barrier in vitro and their influence on barrier integrity. Biochem Biophys Res Commun 406(1):64–69. doi:10.1016/j.bbrc.2011.01.110
Harn HJ, Lin SZ, Lin PC, Liu CY, Liu PY, Chang LF, Yen SY, Hsieh DK, Liu FC, Tai DF, Chiou TW (2011) Local interstitial delivery of z-butylidenephthalide by polymer wafers against malignant human gliomas. Neuro Oncol 13(6):635–648. doi:10.1093/neuonc/nor021
Storm PB, Moriarity JL, Tyler B, Burger PC, Brem H, Weingart J (2002) Polymer delivery of camptothecin against 9L gliosarcoma: release, distribution, and efficacy. J Neurooncol 56(3):209–217
Rapoport SI (2001) Advances in osmotic opening of the blood-brain barrier to enhance CNS chemotherapy. Expert Opin Investig Drugs 10(10):1809–1818. doi:10.1517/13543784.10.10.1809
Zylber-Katz E, Gomori JM, Schwartz A, Lossos A, Bokstein F, Siegal T (2000) Pharmacokinetics of methotrexate in cerebrospinal fluid and serum after osmotic blood-brain barrier disruption in patients with brain lymphoma. Clin Pharmacol Ther 67(6):631–641. doi:10.1067/mcp.2000.106932
Gabathuler R (2010) Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol Dis 37(1):48–57. doi:10.1016/j.nbd.2009.07.028
Kida H, Fujii M, Inoue T, He Y, Maruta Y, Nomura S, Taniguchi K, Ichikawa T, Saito T, Yamakawa T, Suzuki M (2012) Focal brain cooling terminates the faster frequency components of epileptic discharges induced by penicillin G in anesthetized rats. Clin Neurophysiol 123(9):1708–1713. doi:10.1016/j.clinph.2012.02.074
Fujioka H, Fujii M, Koizumi H, Imoto H, Nomura S, Saito T, Yamakawa T, Suzuki M (2010) An implantable, focal brain cooling device suppresses nociceptive pain in rats. Neurosci Res 66(4):402–405. doi:10.1016/j.neures.2009.12.014
Imoto H, Fujii M, Uchiyama J, Fujisawa H, Nakano K, Kunitsugu I, Nomura S, Saito T, Suzuki M (2006) Use of a Peltier chip with a newly devised local brain-cooling system for neocortical seizures in the rat technical note. J Neurosurg 104(1):150–156. doi:10.3171/jns.2006.104.1.150
Fujii M, Inoue T, Nomura S, Maruta Y, He Y, Koizumi H, Shirao S, Owada Y, Kunitsugu I, Yamakawa T, Tokiwa T, Ishizuka S, Suzuki M (2012) Cooling of the epileptic focus suppresses seizures with minimal influence on neurologic functions. Epilepsia 53(3):485–493. doi:10.1111/j.1528-1167.2011.03388.x
Yenari MA, Han HS (2012) Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci 13(4):267–278. doi:10.1038/nrn3174
Natah SS, Srinivasan S, Pittman Q, Zhao Z, Dunn JF (2009) Effects of acute hypoxia and hyperthermia on the permeability of the blood-brain barrier in adult rats. J Appl Physiol 107(4):1348–1356. doi:10.1152/japplphysiol.91484.2008
Montesano R, Pepper MS, Mohle-Steinlein U, Risau W, Wagner EF, Orci L (1990) Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell 62(3):435–445. doi:10.1016/0092-8674(90)90009-4
Oku T, Fujii M, Tanaka N, Imoto H, Uchiyama J, Oka F, Kunitsugu I, Fujioka H, Nomura S, Kajiwara K, Fujisawa H, Kato S, Saito T, Suzuki M (2009) The influence of focal brain cooling on neurophysiopathology: validation for clinical application. J Neurosurg 110(6):1209–1217. doi:10.3171/2009.1.JNS08499
Ohtsuki S, Yamaguchi H, Katsukura Y, Asashima T, Terasaki T (2008) mRNA expression levels of tight junction protein genes in mouse brain capillary endothelial cells highly purified by magnetic cell sorting. J Neurochem 104(1):147–154. doi:10.1111/j.1471-4159.2007.05008.x
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161(3):653–660. doi:10.1083/jcb.200302070
Kiyatkin EA, Sharma HS (2009) Permeability of the blood-brain barrier depends on brain temperature. Neuroscience 161(3):926–939. doi:10.1016/j.neuroscience.2009.04.004
Kiyatkin EA, Brown PL, Sharma HS (2007) Brain edema and breakdown of the blood-brain barrier during methamphetamine intoxication: critical role of brain hyperthermia. Eur J Neurosci 26(5):1242–1253. doi:10.1111/j.1460-9568.2007.05741.x
Acknowledgments
We thank Prof Takeshi Yamakawa for his critical comments and Miss Ayami Osaki for her excellent technical assistance. This work was supported by a Grant-in-Aid for Specially Promoted Research (No. 20001008 to T.Y. and M.S.), a Grant-in-Aid for Exploratory Research (No. 22659261 to M.S.), and a Grant-in-Aid for Scientific Research (C) (No. 21590215 to Y.O.) from The Ministry of Education, Culture, Sports, Science and Technology (MEXT), and by the Yamaguchi University Research Project on STRESS.
Conflict of interest
The authors report no conflict of interest concerning the materials and methods used in this study or the findings specified in this paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2013_1066_MOESM1_ESM.pdf
Restored claudin-5 expression on membrane protein fraction after rewarming. Western blot analysis of membrane protein fraction from bEnd3 cells revealed that a claudin-5 expression was decreased after the cells were cooled at 15 °C for 1 h (1 h) and restored to its original level 1 h after rewarming to 37 °C (+1 h). The expression level of membranous claudin-5 remained for the next 24 h (+24 h). Na–K-ATPase was used as the internal control for membranous protein fraction. Three independent experiments were performed and representative data were shown (PDF 34 kb)
Rights and permissions
About this article
Cite this article
Inamura, A., Adachi, Y., Inoue, T. et al. Cooling Treatment Transiently Increases the Permeability of Brain Capillary Endothelial Cells Through Translocation of Claudin-5. Neurochem Res 38, 1641–1647 (2013). https://doi.org/10.1007/s11064-013-1066-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1066-4