Abstract
Purpose
Gliomagenesis and resistance of glioblastoma (GBM) are believed to be mediated by glioma stem cells (GSC). Evidence suggests that SHH signaling promotes GSC proliferation and self-renewal.
Methods
ABTC-0904 was a two-arm, multicenter phase 0/II study of GDC-0449, an oral inhibitor of Smoothened (SMO) in patients undergoing resection for recurrent GBM. All patients (Arms I and II) had surgery and received drug post-operatively. Only patients in Arm I received drug prior to surgery. The primary objective was to determine 6-month progression free survival (PFS-6). Secondary endpoints include median PFS (mPFS) and overall survival (mOS), response rate, and toxicity. Correlative studies included bioanalysis of GDC-0449, and inhibition of SHH signaling, GSC proliferation and self-renewal.
Results
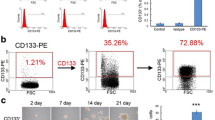
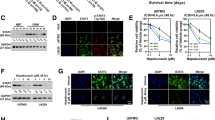
Forty-one patients were enrolled. Pharmacokinetics of GDC-0449 in plasma demonstrated levels within expected therapeutic range in 75% of patients. The proportion of tumorcells producing CD133+ neurospheres, neurosphere proliferation, self-renewal, and expression of the SHh downstream signaling was significantly decreased in Arm I following GDC-0449 treatment (p < 0.005; p < 0.001 respectively) compared to Arm II (no drug pre-op). Treatment was well tolerated. There were no objective responders in either arm. Overall PFS-6 was 2.4% (95% CI 0.9–11.1%). Median PFS was 2.3 months (95% CI 1.9–2.6) and mOS was 7.8 months (95% CI 5.4–10.1).
Conclusions
GDC-0449 was well tolerated, reached tumor, and inhibited CD133+ neurosphere formation, but had little clinical efficacy as a single agent in rGBM. This suggests growth and maintenance of rGBM is not solely dependent on the SHH pathway thus targeting SMO may require combined approaches.



Similar content being viewed by others
Data availability statement
All data refered to in this manuscript is available for review.
References
Singh SK, Clarke ID, Terasaki M et al (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63(18):5821–5828
Singh SK, Hawkins C, Clarke ID et al (2004) Identification of human brain tumour initiating cells. Nature 432(7015):396–401. https://doi.org/10.1038/nature03128
Yuan X, Curtin J, Xiong Y et al (2004) Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene 23(58):9392–9400. https://doi.org/10.1038/sj.onc.1208311
Dirks PB (2008) Brain tumor stem cells: bringing order to the chaos of brain cancer. J Clin Oncol 26(17):2916–2924. https://doi.org/10.1200/JCO.2008.17.6792
Liu G, Yuan X, Zeng Z et al (2006) Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 2(5):67. https://doi.org/10.1186/1476-4598-5-67
Beachy PA, Karhadkar SS, Berman DM (2004) Tissue repair and stem cell renewal in carcinogenesis. Nature 432(7015):324–331. https://doi.org/10.1038/nature03100
Bar EE, Chaudhry A, Lin A et al (2007) Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells 25(10):2524–2533. https://doi.org/10.1634/stemcells.2007-0166
Clement V, Sanchez P, de Tribolet N, Radovanovic I, RuiziAltaba A (2007) HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol 17(2):165–172. https://doi.org/10.1016/j.cub.2006.11.033
Von Hoff DD, LoRusso PM, Rudin CM et al (2009) Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med 361(12):1164–1172. https://doi.org/10.1056/NEJMoa0905360
Rudin CM, Hann CL, Laterra J et al (2009) Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med 361(12):1173–1178. https://doi.org/10.1056/NEJMoa0902903
Graham RA, Lum BL, Cheeti S et al (2011) Pharmacokinetics of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors: the role of alpha-1-acid glycoprotein binding. Clin Cancer Res 17(8):2512–2520. https://doi.org/10.1158/1078-0432.CCR-10-2736
Hjelmeland AB, WuQ HJ et al (2011) Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ 18(5):529–540
Guryanova OA, Wu Q, Cheng L et al (2011) Nonreceptor tyrosine kinease BMX maintains self-renewal, and tumorigenic potential of gliomblastoma stem cells by activating STAT3. Cancer Cell 19(4):498–511
Eyler CE, Wu W, Yan K et al (2011) Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell 146(1):53–66
Heddleston JM, Wu Q, Rivera M et al (2012) Hypoxia-induced mixed-lineage leukemia 1 regulates glioma stem cell tumorigenic potential. Cell Death Differ 19:428–439. https://doi.org/10.1038/cdd.2011.109
Kaur H, Phillips-Mason PJ, Burden-Gulley SM et al (2012) Cadherin-11, a marker of the mesenchymal phenotype, regulates glioblastoma cell migration and survival in vivo. Mol Cancer Res 10(3):293–304
Huang P, Sandhya R, Ahluwalia MS et al (2012) Tumor necrosis factor receptor I expression on tumor-associated endothelial cells in glioblastomas provides a pro-apoptotic signal that is negatively regulated by integrin a681. Cancer Res 72(6):1428–1437
Lathia J, Li M, Hall PE et al (2012) Laminin alpha 2 enables glioblastoma stem cell growth. Ann Neurol 72(5):766–778. https://doi.org/10.1002/ana.23674
Jin X, Kim JLY, Wu A et al (2017) Targeting glioma stem cells through combined BMI1 and EZH2 inhibition. Nat Med 23:1352–1361. https://doi.org/10.1038/nm.4415
Li Z, Bao S, Wu Q et al (2009) Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15(6):501–513
Wang J, Wang H, Li Z et al (2008) c-Myc is required for maintenance of glioma cancer stem cells. PLoS ONE 3(11):e3769
Lathia JD, Gallagher J, Heddleston JM et al (2010) Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 6(5):421–432
Esteller M, Hamilton SR, Burger PC et al (1999) Inactivation of the DNA repair gene 06-methylguanineDNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res 59:793–797
Verhaak RGW, Hoadley KA, Purdom E et al (2010) An integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR and NF1. Cancer Cell 17(1):98–110
Phillips HS, Kharbanda S, Chen R et al (2006) Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression and resemble stages in neurogenesis. Cancer Cell 9:157–173
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Wong H, Alicke B, West KA et al (2011) Pharmacokinetic-pharmacodynamic analysis of vismodegib in preclinical models of mutational and ligand-dependent Hedgehog pathway activation. Clin Cancer Res 17(14):4682–4692. https://doi.org/10.1158/1078-0432.CCR-11-0975
LoRusso PM, Rudin CM, Reddy JC et al (2011) Phase I trial of Hedgehog pathway inhibitor GDC-0449 (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res 17(8):2052–2511
Chen J, Li Y, Yu TS et al (2012) A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488(7412):522–526. https://doi.org/10.1038/nature11287
Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V et al (2012) Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell 22(3):373–388. https://doi.org/10.1016/j.ccr.2012.07.016
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996. https://doi.org/10.1056/NEJMoa043330
Nabors LB, Fiveash J (2005) Treatment of adults with recurrent malignant glioma. Expert Rev Neurother 5(4):509–514. https://doi.org/10.1586/14737175.5.4.509
Pitz MW, Desai A, Grossman SA, Blakeley JO (2011) Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neuroncol 104(3):629–638. https://doi.org/10.1007/s11060-011-0564-y
Hoashi T, Kanda N, Saeki H (2022) Molecular mechanisms and targeted therapies of advanced basal cell carcinoma. Int J Mol Sci 23(19):11968. https://doi.org/10.3390/ijms231911968
Acknowledgements
The authors acknowledge the contributions of the patients who participated in this study and their families. We thank Lisa Rogers, DO, formerly from the Seidman Cancer Center, John Pink, Ph.D., and Erin Hohler of the Translational Research Core of the Case Comprehensive Cancer Center (CCCC) for coordinating the biomarker studies, as well as Megan Sims, of ABTC for clinical coordination.
Funding
National Institutes of Health [CA 137443-ARRA]; The Adult Brain Tumor Consortium (ABTC; PI, S.G.; Co-I, A.E.S); The Peter D. Cristal Chair; The Kimble Foundation; The Ferry Family Foundation; The Kaufman Fund (to A.E.S.).
Author information
Authors and Affiliations
Contributions
Study conception and design: SG, AES, MP, NT, WT. Clinical Protocol: AES, CJN, and SG. Development of Biomarker Studies: AES. Surgical Training of investigators: AES. Statistical design of study: Lamborn, XY. Conduct of clinical studies: CJN, AES, SG, MP. Performance of PK and PD studies: Graham, Supko. Performance of neurosphere assays, PCR, and microscopy: AES, AK-F. Analysis and interpretation of data: AES, CJN, Barnholtz-Sloan, AK-F, XY, Rich, SG, Prados. Drafting the article: AES, MP, Nock, Rich, SG. Critically revising the article: all authors. Patient care: AES, CJN, SG, MP.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests.
Ethical approval
Authors of research involving human or animal subjects should include a statement that confirms that the study was approved (or granted exemption) by the appropriate institutional and/or national research ethics committee (including the name of the ethics committee and reference number, if available)…
Consent to participate
For all research involving human subjects, freely-given, informed consent to participate in the study must be obtained from participants (or their parent or legal guardian in the case of children under 16) and a statement to this effect should appear in the manuscript.
Informed consent
Informed consent was obtained from all individual participants included in the study. Written informed consent was obtained from the parents.
Consent to publish
Individuals may consent to participate in a study, but object to having their data published in a journal article. If your manuscript contains any individual person’s data in any form (including any individual details, images or videos), consent for publication must be obtained from that person, or in the case of children, their parent or legal guardian. This is in particular applicable to case studies. A statement confirming that consent to publish has been received from all participants should appear in the manuscript.
Research involving human and animal rights
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 1a–c.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sloan, A.E., Nock, C.J., Ye, X. et al. ABTC-0904: targeting glioma stem cells in GBM: a phase 0/II study of hedgehog pathway inhibitor GDC-0449. J Neurooncol 161, 33–43 (2023). https://doi.org/10.1007/s11060-022-04193-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04193-3