Abstract
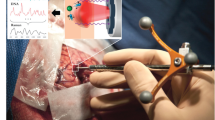
Surgical excision of brain tumors provides a means of cytoreduction and diagnosis while minimizing neurologic deficit and improving overall survival. Despite advances in functional and three-dimensional stereotactic navigation and intraoperative magnetic resonance imaging, delineating tissue in real time with physiological confirmation is challenging. Raman spectroscopy is a promising investigative and diagnostic tool for neurosurgery, which provides rapid, non-destructive molecular characterization in vivo or in vitro for biopsy, margin assessment, or laboratory uses. The Raman Effect occurs when light temporarily changes a bond’s polarizability, causing change in the vibrational frequency, with a corresponding change in energy/wavelength of the scattered photon. The recorded inelastic scattering results in a “fingerprint” or Raman spectrum of the constituent under investigation. The amount, location, and intensity of peaks in the fingerprint vary based on the amount of vibrational bonds in a molecule and their ensemble interactions with each other. Distinct differences between various pathologic conditions are shown as different intensities of the same peak, or shifting of a peak based on the binding conformation. Raman spectroscopy has potential for integration into clinical practice, particularly in distinguishing normal and diseased tissue as an adjunct to standard pathologic diagnosis. Further, development of fiber-optic Raman probes that fit through the instrument port of a standard endoscope now allows researchers and clinicians to utilize spectroscopic information for evaluation of in vivo tissue. This review highlights the need for such an instrument, summarizes neurosurgical Raman work performed to date, and discusses the future applications of neurosurgical Raman spectroscopy.


Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F (2013) GLOBOCAN 2012 v1. 0, cancer incidence and mortality worldwide: IARC CancerBase No. 11 [internet]. International Agency for Research on Cancer, Lyon. globocan IARC FR (Accessed 10 Oct 2014)
Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE (2014) Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol 32:774–782
Sanai N, Polley M-Y, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas: clinical article. J Neurosurg 115:3–8
Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgård G, Solheim O (2012) Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA 308:1881–1888
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen H-J (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Wu J-S, Zhou L-F, Tang W-J, Mao Y, Hu J, Song Y-Y, Hong X-N, Du G-H (2007) Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery 61:935–949
Coburger J, Scheuerle A, Kapapa T, Engelke J, Thal DR, Wirtz CR, König R (2015) Sensitivity and specificity of linear array intraoperative ultrasound in glioblastoma surgery: a comparative study with high field intraoperative MRI and conventional sector array ultrasound. Neurosurg Rev 38:499–509
Prada F, Perin A, Martegani A, Aiani L, Solbiati L, Lamperti M, Casali C, Legnani F, Mattei L, Saladino A (2014) Intraoperative contrast-enhanced ultrasound for brain tumor surgery. Neurosurgery 74:542–552
Selbekk T, Jakola AS, Solheim O, Johansen TF, Lindseth F, Reinertsen I, Unsgård G (2013) Ultrasound imaging in neurosurgery: approaches to minimize surgically induced image artefacts for improved resection control. Acta Neurochir 155:973–980
Chang EF, Clark A, Smith JS, Polley M-Y, Chang SM, Barbaro NM, Parsa AT, McDermott MW, Berger MS (2011) Functional mapping–guided resection of low-grade gliomas in eloquent areas of the brain: improvement of long-term survival. J Neurosurg 114:566–573
De Witt HPC, Robles SG, Zwinderman AH, Duffau H, Berger MS (2012) Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol 30:2559–2565
Stummer W, Tonn J-C, Mehdorn HM, Nestler U, Franz K, Goetz C, Bink A, Pichlmeier U (2011) Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study: clinical article. J Neurosurg 114:613–623
Li Y, Rey-Dios R, Roberts DW, Valdés PA, Cohen-Gadol AA (2014) Intraoperative fluorescence-guided resection of high-grade gliomas: a comparison of the present techniques and evolution of future strategies. World Neurosurg 82:175–185
Santagata S, Eberlin LS, Norton I, Calligaris D, Feldman DR, Ide JL, Liu X, Wiley JS, Vestal ML, Ramkissoon SH (2014) Intraoperative mass spectrometry mapping of an onco-metabolite to guide brain tumor surgery. Proc Natl Acad Sci 111:11121–11126
Butte PV, Mamelak AN, Nuno M, Bannykh SI, Black KL, Marcu L (2011) Fluorescence lifetime spectroscopy for guided therapy of brain tumors. Neuroimage 54:S125–S135
Sun Y, Hatami N, Yee M, Phipps J, Elson DS, Gorin F, Schrot RJ, Marcu L (2010) Fluorescence lifetime imaging microscopy for brain tumor image-guided surgery. J Biomed Opt 15:056022–056025
Butte PV, Mamelak A, Parrish-Novak J, Drazin D, Shweikeh F, Gangalum PR, Chesnokova A, Ljubimova JY, Black K (2014) Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurg Focus 36(2):E1
Shinoda J, Yano H, Yoshimura S-I, Okumura A, Kaku Y, Iwama T, Sakai N (2003) Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium: technical note. J Neurosurg 99:597–603
Orringer DA, Koo Y-EL, Chen T, Kim G, Hah HJ, Xu H, Wang S, Keep R, Philbert MA, Kopelman R (2009) In vitro characterization of a targeted, dye-loaded nanodevice for intraoperative tumor delineation. Neurosurgery 64:965–971
Raman C (1928) A new radiation. Indian J Phys 2:11
Chase B (1994) A new generation of Raman instrumentation. Appl Spectrosc 48(7):14A–19A
Campbell DPR, White JR (2000) Polymer characterization: physical techniques, 2nd edn. Stanley Thornes Publishers Ltd
Caroline ML, Vasudevan S (2009) Growth and characterization of l-phenylalanine nitric acid, a new organic nonlinear optical material. Materials Lett 63:41–44
Movasaghi Z, Rehman S, Rehman IU (2007) Raman spectroscopy of biological tissues. Applied Spectrosc Rev 42:493–541
Mayo DW, Miller FA, Hannah RW (2004) Course notes on the interpretation of infrared and Raman spectra. Wiley Online Library, New York. http://onlinelibrary.wiley.com/book/10.1002/0471690082
Nelson DL, Lehninger AL, Cox MM (2005) Lehninger principles of biochemistry, 4th edn. W. H. Freeman and Company, New York
Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. J Gen Physiol 8:519–530
Tashibu K (1990) Analysis of water content in rat brain using Raman spectroscopy. No To Shinkei 42:999–1004
Kast RE, Tucker SC, Killian K, Trexler M, Honn KV, Auner GW (2014) Emerging technology: applications of Raman spectroscopy for prostate cancer. Cancer Metastasis Rev 33:673–693
Mizuno A, Kitajima H, Kawauchi K, Muraishi S, Ozaki Y (1994) Near-infrared Fourier transform Raman spectroscopic study of human brain tissues and tumours. J Raman Spectrosc 25:25–29
Koljenovic S, Schut TB, Wolthuis Rd, De Jong B, Santos L, Caspers PJ, Kros JM, Puppels GJ (2005) Tissue characterization using high wave number Raman spectroscopy. J Biomed Opt 10:031116–03111611
Koljenovic S, Schut T, Vincent A, Kros J, Puppels G (2005) Detection of meningioma in dura mater by Raman spectroscopy. Anal Chem 77:7958–7965
Krafft C, Miljanic S, Sobottka SB, Schackert G, Salzer R (2003) Near infrared Raman spectroscopy to study the composition of human brain tissue and tumors. In: Wagnieres G (ed) Diagnostic optical spectroscopy in biomedicine II: proceedings of SPIE, vol 5141. SPIE, Munich, pp 230–236
Campanella R (1991) Membrane lipids modifications in human gliomas of different degree of malignancy. J Neurosurg Sci 36:11–25
Zhang C, Moore LM, Li X, Yung WA, Zhang W (2013) IDH1/2 mutations target a key hallmark of cancer by deregulating cellular metabolism in glioma. Neuro oncol 15:1114–1126
Gajjar K, Heppenstall LD, Pang W, Ashton KM, Trevisan J, Patel II, Llabjani V, Stringfellow HF, Martin-Hirsch PL, Dawson T (2013) Diagnostic segregation of human brain tumours using Fourier-transform infrared and/or Raman spectroscopy coupled with discriminant analysis. Anal Methods 5:89–102
Bergholt M, Zheng W, Lin K, Ho K, Teh M, Yeoh K, So JY, Huang Z (2011) In vivo diagnosis of esophageal cancer using image-guided Raman endoscopy and biomolecular modeling. Technol Cancer Res Treat 10:103–112
Bergholt MS, Zheng W, Lin K, Ho KY, Teh M, Yeoh KG, So JBY, Huang Z (2010) Raman endoscopy for in vivo differentiation between benign and malignant ulcers in the stomach. Analyst 135:3162–3168
Duraipandian S, Mo J, Zheng W, Huang Z (2014) Near-infrared Raman spectroscopy for assessing biochemical changes of cervical tissue associated with precarcinogenic transformation. Analyst 139:5379–5386
Haka AS, Volynskaya Z, Gardecki JA, Nazemi J, Lyons J, Hicks D, Fitzmaurice M, Dasari RR, Crowe JP, Feld MS (2006) In vivo margin assessment during partial mastectomy breast surgery using Raman spectroscopy. Cancer Res 66:3317–3322
Banerjee H, Zhang L (2007) Deciphering the finger prints of brain cancer astrocytoma in comparison to astrocytes by using near infrared Raman spectroscopy. Mol Cell Biochem 295:237–240
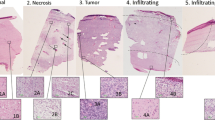
Kalkanis SN, Kast RE, Rosenblum ML, Mikkelsen T, Yurgelevic SM, Nelson KM, Raghunathan A, Poisson LM, Auner GW (2014) Raman spectroscopy to distinguish grey matter, necrosis, and glioblastoma multiforme in frozen tissue sections. J Neurooncol 116:477–485
Kast R, Auner G, Rosenblum M, Mikkelsen T, Yurgelevic S, Raghunathan A, Poisson L, Kalkanis S (2014) Raman molecular imaging of brain frozen tissue sections. J Neurooncol 120:55–62
Kast R, Auner G, Yurgelevic S, Broadbent B, Raghunathan A, Poisson LM, Mikkelsen T, Rosenblum ML, Kalkanis SN (2015) Identification of regions of normal grey matter and white matter from pathologic glioblastoma and necrosis in frozen sections using Raman imaging. J Neurooncol 125:287–295
Tanahashi K, Natsume A, Ohka F, Momota H, Kato A, Motomura K, Watabe N, Muraishi S, Nakahara H, Saito Y (2014) Assessment of tumor cells in a mouse model of diffuse infiltrative glioma by Raman spectroscopy. Biomed Res Int 2014:860241.
Desroches J, Jermyn M, Mok K, Lemieux-Leduc C, Mercier J, St-Arnaud K, Urmey K, Guiot M-C, Marple E, Petrecca K (2015) Characterization of a Raman spectroscopy probe system for intraoperative brain tissue classification. Biomed Opt Express 6:2380–2397
Jermyn M, Mok K, Mercier J, Desroches J, Pichette J, Saint-Arnaud K, Bernstein L, Guiot M-C, Petrecca K, Leblond F (2015) Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci Transl Med 7:274ra219
Verisante Technology, Inc. Announces Brain Tumour Study in the UK (2015). Verisante press release 2015
Xie W, Schlucker S (2013) Medical applications of surface-enhanced Raman scattering. Phys Chem Chem Phys 15:5329–5344
Buckley K, Kerns JG, Parker AW, Goodship AE, Matousek P (2014) Decomposition of in vivo spatially offset Raman spectroscopy data using multivariate analysis techniques. J Raman Spectrosc 45:188–192
Keller MD, Wilson RH, Mycek M-A, Mahadevan-Jansen A (2010) Monte Carlo model of spatially offset Raman spectroscopy for breast tumor margin analysis. Appl Spectrosc 64:607–614
Zumbusch A, Müller M (2007) Coherent anti-Stokes Raman scattering microscopy. Chem Phys Chem 8:2156–2170
Freudiger CW, Min W, Saar BG, Lu S, Holtom GR, He C, Tsai JC, Kang JX, Xie XS (2008) Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 322(5909):1857–1861
Freudiger CW, Xie XS (2011) In vivo imaging with stimulated Raman scattering microscopy. Opt Photon News 22:27–27
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflicts of interest.
Additional information
Brandy Broadbent and James Tseng have contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Broadbent, B., Tseng, J., Kast, R. et al. Shining light on neurosurgery diagnostics using Raman spectroscopy. J Neurooncol 130, 1–9 (2016). https://doi.org/10.1007/s11060-016-2223-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2223-9