Abstract
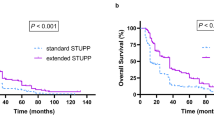
The goal of this meta-analysis was to identify the temozolomide (TMZ) regimen with optimal efficacy and tolerance for treatment of recurrent high-grade glioma (HGG). The PubMed and EMBASE databases were searched from the earliest records to February 2015, which identified 33 studies with 1760 participants that met the inclusion criteria. The standard schedule and three most common dose-dense regimens of TMZ therapy for recurrent HGG were included in this meta-analysis. The schedule of 7 days on/7 days off for the treatment of grade IV gliomas was significantly superior to the standard regimen with respect to progression-free survival at 6 months (34.8 %; 95 % confidence interval (CI) 27.0–43.4 %) and 12 months (15.5 %; 95 % CI 10.7–21.8 %). For grade III gliomas, this regimen conveyed a significantly greater overall survival (OS) rate at 12 months (79.0 %; 95 % CI 56.2–91.7 %), as compared to the standard schedule. Also, the 21 days on/7 days off regimen had significantly longer OS rates at 6 months (73.6 %; 95 % CI 63.4–81.8 %) and 12 months (40.6 %; 95 % CI 32.6–48.6 %) than the standard regimen for grade IV gliomas. In addition, the standard schedule showed a significantly higher clinical benefit rate than the 7 days on/7 days off and 21 days on/7 days off regimens. However, the grade 3–4 toxicity rate of lymphopenia of the standard schedule was 76.5 % (95 % CI 45.5–92.7 %), which was the highest among the four regimens. Recurrent HGG patients receiving personalized treatment should be closely followed up, especially those with concurrent hematological diseases.


Similar content being viewed by others
References
Kong DS, Lee JI, Kim WS et al (2006) A pilot study of metronomic temozolomide treatment in patients with recurrent temozolomide-refractory glioblastoma. Oncol Rep 16:1117–1121
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. doi:10.1007/s00401-007-0243-4
Easaw JC, Mason WP, Perry J et al (2011) Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol 18:e126–e136
Gilbert MR (2006) Advances in the treatment of primary brain tumors: dawn of a new era? Curr Oncol Rep 8:45–49
Stupp R, Mason WP, Van Den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Han SJ, Rolston JD, Molinaro AM et al (2014) Phase II trial of 7 days on/7 days off temozolmide for recurrent high-grade glioma. Neuro Oncol 16:1255–1262. doi:10.1093/neuonc/nou044
Terasaki M, Tokutomi T, Shigemori M (2009) Salvage therapy with temozolomide for recurrent or progressive high-grade gliomas refractory to ACNU [1-(4-amino-2-methyl-5-pyrimidynyl) methyl-3-(2-chloroethyl)-3-nitrosourea hydrochloride]. Mol Med Rep 2:417–421. doi:10.3892/mmr_00000115
Norden AD, Lesser GJ, Drappatz J et al (2013) Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro Oncol 15:930–935. doi:10.1093/neuonc/not040
Omuro A, Chan TA, Abrey LE et al (2013) Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol 15:242–250. doi:10.1093/neuonc/nos295
Yung WK, Albright RE, Olson J et al (2000) A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 83:588–593. doi:10.1054/bjoc.2000.1316
Wick W, Platten M, Weller M (2009) New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol 11:69–79. doi:10.1215/15228517-2008-078
Newlands ES, Stevens MF, Wedge SR et al (1997) Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev 23:35–61
Tisdale MJ (1987) Antitumor imidazotetrazines–XV. Role of guanine O6 alkylation in the mechanism of cytotoxicity of imidazotetrazinones. Biochem Pharmacol 36:457–462
Gerson SL (2002) Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol 20:2388–2399
Kurzen H, Schmitt S, Näher H et al (2003) Inhibition of angiogenesis by non-toxic doses of temozolomide. Anticancer Drugs 14:515–522
Kim JT, Kim J-S, Ko KW et al (2006) Metronomic treatment of temozolomide inhibits tumor cell growth through reduction of angiogenesis and augmentation of apoptosis in orthotopic models of gliomas. Oncol Rep 16:33–39
Klement G, Huang P, Mayer B et al (2002) Differences in therapeutic indexes of combination metronomic chemotherapy and an anti-VEGFR-2 antibody in multidrug-resistant human breast cancer xenografts. Clin Cancer Res 8:221–232
Plate KH, Risau W (1995) Angiogenesis in malignant gliomas. Glia 15:339–347
Tolcher A, Gerson S, Denis L et al (2003) Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer 88:1004–1011
Chen C, Xu T, Lu Y et al (2013) The efficacy of temozolomide for recurrent glioblastoma multiforme. Eur J Neurol 20:223–230. doi:10.1111/j.1468-1331.2012.03778.x
Galldiks N, Berhorn T, Blau T et al (2013) “One week on-one week off”: efficacy and side effects of dose-intensified temozolomide chemotherapy: experiences of a single center. J Neurooncol 112:209–215. doi:10.1007/s11060-013-1048-z
Moher D, Liberati A, Tetzlaff J et al (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336–341. doi:10.1016/j.ijsu.2010.02.007
Macdonald DR, Cascino TL, Schold SC et al (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Colditz GA, Burdick E, Mosteller F (1995) Heterogeneity in meta-analysis of data from epidemiologic studies: a commentary. Am J Epidemiol 142:371–382
Higgins J, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Harris R, Bradburn M, Deeks J et al (2008) Metan: fixed- and random-effects meta-analysis. Stata J 8:3–28
Hassler M, Micksche M, Stockhammer G et al (2006) Temozolomide for recurrent or progressive high-grade malignant glioma: results of an Austrian multicenter observational study. Wien Klin Wochenschr 118:230–238. doi:10.1007/s00508-006-0576-3
Chang SM, Theodosopoulos P, Lamborn K et al (2004) Temozolomide in the treatment of recurrent malignant glioma. Cancer 100:605–611. doi:10.1002/cncr.11949
Aoki T, Mizutani T, Ishikawa M et al (2003) A first feasibility study of temozolomide for Japanese patients with recurrent anaplastic astrocytoma and glioblastoma multiforme. Int J Clin Oncol 8:301–304. doi:10.1007/s10147-003-0339-3
Brandes AA, Ermani M, Basso U et al (2002) Temozolomide in patients with glioblastoma at second relapse after first line nitrosourea-procarbazine failure a phase II study. Oncology 63:38–41
Chinot OL, Honore S, Dufour H et al (2001) Safety and efficacy of temozolomide in patients with recurrent anaplastic oligodendrogliomas after standard radiotherapy and chemotherapy. J Clin Oncol 19:2449–2455
Harris MT, Rosenthal MA, Ashley DL et al (2001) An Australian experience with temozolomide for the treatment of recurrent high grade gliomas. J Clin Neurosci 8:325–327. doi:10.1054/jocn.2000.0809
Brandes AA, Ermani M, Basso U et al (2001) Temozolomide as a second-line systemic regimen in recurrent high-grade glioma, a phase II study. Ann Oncol 12:255–257
Brada M, Hoang-Xuan K, Rampling R et al (2001) Multicenter phase II trial of temozolomide in patients with glioblastoma. Ann Oncol 12:259–266
Paulsen F, Hoffmann W, Becker G et al (1999) Chemotherapy in the treatment of recurrent glioblastoma multiforme: ifosfamide versus temozolomide. J Cancer Res Clin Oncol 125:411–418
Bower M, Newlands ES, Bleehen NM et al (1997) Multicentre CRC phase II trial of temozolomide in recurrent or progressive high-grade glioma. Cancer Chemother Pharmacol 40:484–488
Yang SH, Kim MK, Lee TK et al (2006) Temozolomide chemotherapy in patients with recurrent malignant gliomas. J Korean Med Sci 21:739–744
Teixeira MM, Garcia I, Portela I et al (2002) Temozolomide in second-line treatment after prior nitrosurea-based chemotherapy in glioblastoma multiforme: experience from a Portuguese institution. Int J Clin Pharmacol Res 22:19–22
Perry JR, Belanger K, Mason WP et al (2010) Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol 28:2051–2057. doi:10.1200/jco.2009.26.5520
Perry JR, Rizek P, Cashman R et al (2008) Temozolomide rechallenge in recurrent malignant glioma by using a continuous temozolomide schedule: the “rescue” approach. Cancer 113:2152–2157. doi:10.1002/cncr.23813
Kong DS, Lee JI, Kim JH et al (2010) Phase II trial of low-dose continuous (metronomic) treatment of temozolomide for recurrent glioblastoma. Neuro Oncol 12:289–296. doi:10.1093/neuonc/nop030
Strik HM, Buhk JH, Wrede A et al (2008) Rechallenge with temozolomide with different scheduling is effective in recurrent malignant gliomas. Mol Med Rep 1:863–867. doi:10.3892/mmr_00000042
Brandes AA, Tosoni A, Cavallo G et al (2006) Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from gruppo italiano cooperativo di neuro-oncologia (GICNO). Br J Cancer 95:1155–1160. doi:10.1038/sj.bjc.6603376
Berrocal A, Perez Segura P, Gil M et al (2010) Extended-schedule dose-dense temozolomide in refractory gliomas. J Neurooncol 96:417–422. doi:10.1007/s11060-009-9980-7
Neyns B, Chaskis C, Joosens E et al (2008) A multicenter cohort study of dose-dense temozolomide (21 of 28 days) for the treatment of recurrent anaplastic astrocytoma or oligoastrocytoma. Cancer Invest 26:269–277. doi:10.1080/07357900701708393
Santoni M, Paccapelo A, Burattini L et al (2012) Protracted low doses of temozolomide for the treatment of patients with recurrent glioblastoma: a phase II study. Oncol Lett 4:799–801. doi:10.3892/ol.2012.788
Taal W, Segers-van Rijn JM, Kros JM et al (2012) Dose dense 1 week on/1 week off temozolomide in recurrent glioma: a retrospective study. J Neurooncol 108:195–200. doi:10.1007/s11060-012-0832-5
Wick A, Felsberg J, Steinbach JP et al (2007) Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol 25:3357–3361. doi:10.1200/jco.2007.10.7722
Wong S, Rosenthal MA, Dowling A et al (2006) Phase II study of two-weekly temozolomide in patients with high-grade gliomas. J Clin Neurosci 13:18–22. doi:10.1016/j.jocn.2004.10.018
Wick W, Weller M (2005) How lymphotoxic is dose-intensified temozolomide? The glioblastoma experience. J Clin Oncol 23:4235–4236. doi:10.1200/JCO.2004.00.8417
Wick W, Steinbach JP, Kuker WM et al (2004) One week on/one week off: a novel active regimen of temozolomide for recurrent glioblastoma. Neurology 62:2113–2115
Weller M, Tabatabai G, Kästner B et al (2015) MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR Trial. Clin Cancer Res 21:2057–2064
Hegi ME, Diserens A-C, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Esteller M, Garcia-Foncillas J, Andion E et al (2000) Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 343:1350–1354
Krex D, Klink B, Hartmann C et al (2007) Long-term survival with glioblastoma multiforme. Brain 130:2596–2606
Dong X, Liu RY, Chen WD (2014) Correlation of promoter methylation in the MGMT gene with glioma Risk and prognosis: a meta-analysis. Mol Neurobiol. doi:10.1007/s12035-014-8760-3
Weiler M, Hartmann C, Wiewrodt D et al (2010) Chemoradiotherapy of newly diagnosed glioblastoma with intensified temozolomide. Int J Radiat Oncol Biol Phys 77:670–676
Chinot OL, Barrié M, Fuentes S et al (2007) Correlation between O6-methylguanine-DNA methyltransferase and survival in inoperable newly diagnosed glioblastoma patients treated with neoadjuvant temozolomide. J Clin Oncol 25:1470–1475
Kerbel RS, Kamen BA (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4:423–436
Bertolini F, Paul S, Mancuso P et al (2003) Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res 63:4342–4346
Emmenegger U, Man S, Shaked Y et al (2004) A comparative analysis of low-dose metronomic cyclophosphamide reveals absent or low-grade toxicity on tissues highly sensitive to the toxic effects of maximum tolerated dose regimens. Cancer Res 64:3994–4000
Bocci G, Francia G, Man S et al (2003) Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci USA 100:12917–12922
Acknowledgments
We thank all the participants in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have disclosed no conflicts of interest.
Rights and permissions
About this article
Cite this article
Wei, W., Chen, X., Ma, X. et al. The efficacy and safety of various dose-dense regimens of temozolomide for recurrent high-grade glioma: a systematic review with meta-analysis. J Neurooncol 125, 339–349 (2015). https://doi.org/10.1007/s11060-015-1920-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1920-0