Abstract
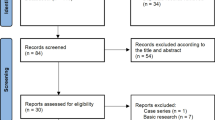
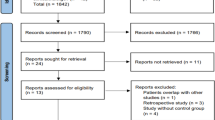
The aim of this study was to assess the effectiveness of adding viral vector-mediated gene therapy with herpes simplex virus thymidine kinase (HSV-tk) to standard treatment, in comparison with standard treatment alone to treat patients with high-grade gliomas (HGGs). A literature search of the databases PubMed, Embase, the Cochrane Library, Web of Science, and Chinese biomedicine was performed to identify eligible studies. Three randomized controlled trials (involving a total of 532 patients) were included in this systematic review. A meta-analysis of included studies demonstrated a significant increase in median survival time (MST) in patients who were treated with HSV-tk gene therapy (mean deviation 0.59, 95 % CI: 0.41–0.76, p < 0.0001). The results of pooled analysis for different patient groups show that overall survival (OS) for all HGG patients was improved by adding gene therapy [hazard ratio (HR) = 0.91, 95 % CI: 0.74–1.13, p = 0.42], while a different result was seen for glioblastoma multiforme (GBM) patients (HR = 1.06, 95 % CI: 0.80–1.41, p = 0.70). Furthermore, the combined results for tumor progression implied that standard therapy was superior to gene therapy [odds ratio (OR) = 1.31, p = 0.09]; yet differences in HR and OR between experimental groups and control groups had no statistical significance (p > 0.05). Based on the best available evidence, it appears that adding gene therapy with HSV-tk has some effect in treating HGG patients, especially with respect to MST. However, neither the pooled analysis of OS, nor the combined analysis of tumor progress indicates any significant advantage to adding gene therapy compared with standard treatment alone. More prospective studies are needed to draw solid conclusions about whether gene therapy has significant prognostic advantage.



Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109
CBTRUS (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2008. Central Brain Tumor Registry of the United States
Davis FG, McCarthy BJ, Berger MS (1999) Centralized databases available for describing primary brain tumor incidence, survival, and treatment: Central Brain Tumor Registry of the United States; surveillance, epidemiology, and end results; and national cancer data base. Neuro Oncol 1(3):205–211
Ma X, Lv Y, Liu J, Wang D, Huang Q, Wang X, Li G, Xu S, Li X (2009) Survival analysis of 205 patients with glioblastoma multiforme: clinical characteristics, treatment and prognosis in China. J Clin Neurosci 16(12):1595–1598. doi:10.1016/j.jocn.2009.02.036
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466. doi:10.1016/s1470-2045(09)70025-7
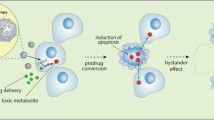
Marsh JC, Goldfarb J, Shafman TD, Diaz AZ (2013) Current status of immunotherapy and gene therapy for high-grade gliomas. Cancer Control 20(1):43–48
Rainov NG, Heidecke V (2011) Clinical development of experimental virus-mediated gene therapy for malignant glioma. Anticancer Agents Med Chem 11(8):739–747
Matuskova M, Hlubinova K, Pastorakova A, Hunakova L, Altanerova V, Altaner C, Kucerova L (2010) HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Lett 290(1):58–67. doi:10.1016/j.canlet.2009.08.028
Chen CY, Chang YN, Ryan P, Linscott M, McGarrity GJ, Chiang YL (1995) Effect of herpes simplex virus thymidine kinase expression levels on ganciclovir-mediated cytotoxicity and the “bystander effect”. Hum Gene Ther 6(11):1467–1476. doi:10.1089/hum.1995.6.11-1467
Rainov NG, Kramm CM, Banning U, Riemann D, Holzhausen HJ, Heidecke V, Burger KJ, Burkert W, Korholz D (2000) Immune response induced by retrovirus-mediated HSV-tk/GCV pharmacogene therapy in patients with glioblastoma multiforme. Gene Ther 21:1853–1858
Chiocca EA, Aguilar LK, Bell SD, Kaur B, Hardcastle J, Cavaliere R, McGregor J, Lo S, Ray-Chaudhuri A, Chakravarti A, Grecula J, Newton H, Harris KS, Grossman RG, Trask TW, Baskin DS, Monterroso C, Manzanera AG, Aguilar-Cordova E, New PZ (2011) Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol 29(27):3611–3619. doi:10.1200/jco.2011.35.5222
Nestler U, Wakimoto H, Siller-Lopez F, Aguilar LK, Chakravarti A, Muzikansky A, Stemmer-Rachamimov A, Chiocca EA, Aguilar-Cordova E, Hochberg FH (2004) The combination of adenoviral HSV TK gene therapy and radiation is effective in athymic mouse glioblastoma xenografts without increasing toxic side effects. J Neurooncol 67(1–2):177–188
Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM (1992) In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science (New York, NY) 256(5063):1550–1552
Izquierdo M, Martin V, de Felipe P, Izquierdo JM, Perez-Higueras A, Cortes ML, Paz JF, Isla A, Blazquez MG (1996) Human malignant brain tumor response to herpes simplex thymidine kinase (HSVtk)/ganciclovir gene therapy. Gene Ther 3(6):491–495
Ram Z, Culver KW, Oshiro EM, Viola JJ, DeVroom HL, Otto E, Long Z, Chiang Y, McGarrity GJ, Muul LM, Katz D, Blaese RM, Oldfield EH (1997) Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med 3(12):1354–1361
Sandmair AM, Loimas S, Puranen P, Immonen A, Kossila M, Puranen M, Hurskainen H, Tyynela K, Turunen M, Vanninen R, Lehtolainen P, Paljarvi L, Johansson R, Vapalahti M, Yla-Herttuala S (2000) Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther 11(16):2197–2205. doi:10.1089/104303400750035726
van Putten EH, Dirven CM, van den Bent MJ, Lamfers ML (2010) Sitimagene ceradenovec: a gene-based drug for the treatment of operable high-grade glioma. Future Oncol (London, England) 6(11):1691–1710. doi:10.2217/fon.10.134
Trask TW, Trask RP, Aguilar-Cordova E, Shine HD, Wyde PR, Goodman JC, Hamilton WJ, Rojas-Martinez A, Chen SH, Woo SL, Grossman RG (2000) Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther 1(2):195–203. doi:10.1006/mthe.2000.0030
Klatzmann D, Valery CA, Bensimon G, Marro B, Boyer O, Mokhtari K, Diquet B, Salzmann JL, Philippon J (1998) A phase I/II study of herpes simplex virus type 1 thymidine kinase “suicide” gene therapy for recurrent glioblastoma. Study Group on Gene Therapy for Glioblastoma. Hum Gene Ther 9(17):2595–2604. doi:10.1089/hum.1998.9.17-2595
Palu G, Cavaggioni A, Calvi P, Franchin E, Pizzato M, Boschetto R, Parolin C, Chilosi M, Ferrini S, Zanusso A, Colombo F (1999) Gene therapy of glioblastoma multiforme via combined expression of suicide and cytokine genes: a pilot study in humans. Gene Ther 6(3):330–337. doi:10.1038/sj.gt.3300805
Shand N, Weber F, Mariani L, Bernstein M, Gianella-Borradori A, Long Z, Sorensen AG, Barbier N (1999) A phase 1-2 clinical trial of gene therapy for recurrent glioblastoma multiforme by tumor transduction with the herpes simplex thymidine kinase gene followed by ganciclovir. GLI328 European-Canadian Study Group. Hum Gene Ther 10(14):2325–2335. doi:10.1089/10430349950016979
Germano IM, Fable J, Gultekin SH, Silvers A (2003) Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neurooncol 65(3):279–289
Prados MD, McDermott M, Chang SM, Wilson CB, Fick J, Culver KW, Van Gilder J, Keles GE, Spence A, Berger M (2003) Treatment of progressive or recurrent glioblastoma multiforme in adults with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration: a phase I/II multi-institutional trial. J Neurooncol 65(3):269–278
Smitt PS, Driesse M, Wolbers J, Kros M, Avezaat C (2003) Treatment of relapsed malignant glioma with an adenoviral vector containing the herpes simplex thymidine kinase gene followed by ganciclovir. Mol Ther 7(6):851–858
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1. 0 [updated March 2011]. The Cochrane Collaboration
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16. doi:10.1186/1745-6215-8-16
Liu JL, Gao W, Kang QM, Zhang XJ, Yang SG (2013) Prognostic value of survivin in patients with gastric cancer: a systematic review with meta-analysis. PloS One 8(8):e71930. doi:10.1371/journal.pone.0071930
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. doi:10.1002/sim.1186
Knobloch K, Yoon U, Vogt PM (2011) Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 39(2):91–92. doi:10.1016/j.jcms.2010.11.001
Rainov NG (2000) A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther 11(17):2389–2401. doi:10.1089/104303400750038499
Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, Langford G, Murray N, Yla-Herttuala S (2004) AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther 10(5):967–972. doi:10.1016/j.ymthe.08.002
Westphal M, Yla-Herttuala S, Martin J, Warnke P, Menei P, Eckland D, Kinley J, Kay R, Ram Z (2013) Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol 14(9):823–833. doi:10.1016/s1470-2045(13)70274-2
Acknowledgments
The authors would like to thank Zheng Li and Bei An (School of Basic Medical Sciences of Lanzhou University, China) for advices on conducting the meta-analysis and writing the article. We also thank two anonymous reviewers and the editor Bharathi Sriram for this article.
Conflicts of interest
The authors indicated no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, F., Tian, J., An, L. et al. Prognostic utility of gene therapy with herpes simplex virus thymidine kinase for patients with high-grade malignant gliomas: a systematic review and meta analysis. J Neurooncol 118, 239–246 (2014). https://doi.org/10.1007/s11060-014-1444-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1444-z