Abstract
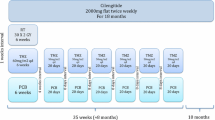
The integrin antagonist cilengitide has been explored as an adjunct with anti-angiogenic properties to standard of care temozolomide chemoradiotherapy (TMZ/RT → TMZ) in newly diagnosed glioblastoma. Preclinical data as well as anecdotal clinical observations indicate that anti-angiogenic treatment may result in altered patterns of tumor progression. Using a standardized approach, we analyzed patterns of progression on MRI in 21 patients enrolled onto a phase 2 trial of cilengitide added to TMZ/RT → TMZ in newly diagnosed glioblastoma. Thirty patients from the experimental treatment arm of the EORTC/NCIC pivotal TMZ trial served as a reference. MRIcro software was used to map location and extent of initial preoperative and recurrent tumors on MRI of both groups into the same stereotaxic space which were then analyzed using an automated tool of image analysis. Clinical and outcome data of the cilengitide-treated patients were similar to those of the EORTC/NCIC trial except for a higher proportion of patients with a methylated O6-methylguanyl-DNA-methyltransferase gene promoter. Analysis of recurrence pattern revealed neither a difference in the size of the recurrent tumor nor in the distance of the recurrences from the preoperative tumor location between groups. Overall frequencies of distant recurrences were 20 % in the reference group and 19 % (4/21 patients) in the cilengitide group. Compared with TMZ/RT → TMZ alone, the addition of cilengitide does not alter patterns of progression. This analysis does not support concerns that integrin antagonism by cilengitide may induce a more aggressive phenotype at progression, but also provides no evidence for an anti-invasive activity of cilengitide in patients with newly diagnosed glioblastoma.
Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SA, Fack F, Thorsen F, Taxt T, Bartos M, Jirik R, Miletic H, Wang J, Stieber D, Stuhr L, Moen I, Rygh CB, Bjerkvig R, Niclou SP (2011) Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci USA 108(9):3749–3754
Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O (2009) Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15(3):220–231
Wick W, Stupp R, Beule AC, Bromberg J, Wick A, Ernemann U, Platten M, Marosi C, Mason WP, van den Bent M, Weller M, Rorden C, Karnath HO (2008) A novel tool to analyze MRI recurrence patterns in glioblastoma. Neuro-Oncology 10(6):1019–1024
Wick A, Dorner N, Schafer N, Hofer S, Heiland S, Schemmer D, Platten M, Weller M, Bendszus M, Wick W (2011) Bevacizumab does not increase the risk of remote relapse in malignant glioma. Ann Neurol 69(3):586–592
Stupp R, Hegi ME, Neyns B, Goldbrunner R, Schlegel U, Clement PM, Grabenbauer GG, Ochsenbein AF, Simon M, Dietrich PY, Pietsch T, Hicking C, Tonn JC, Diserens AC, Pica A, Hermisson M, Krueger S, Picard M, Weller M (2010) Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol 28(16):2712–2718
Rorden C, Brett M (2000) Stereotaxic display of brain lesions. Behav Neurol 12(4):191–200
Wild-Bode C, Weller M, Rimner A, Dichgans J, Wick W (2001) Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res 61(6):2744–2750
Wick W, Wick A, Schulz JB, Dichgans J, Rodemann HP, Weller M (2002) Prevention of irradiation-induced glioma cell invasion by temozolomide involves caspase 3 activity and cleavage of focal adhesion kinase. Cancer Res 62(6):1915–1919
Lamszus K, Kunkel P, Westphal M (2003) Invasion as limitation to anti-angiogenic glioma therapy. Acta Neurochir Suppl 88:169–177
Wick W, Cloughesy F, Nishikawa R, Mason W, Saran F, Henriksson R, Hilton M, Kerloeguen Y, Chinot O (2013) Tumor response based on adapted Macdonald criteria and assessment of pseudoprogression (PsPD) in the phase III AVAglio trial of bevacizumab (Bv) plus temozolomide (T) plus radiotherapy (RT) in newly diagnosed glioblastoma (GBM). J Clin Oncol 31(15 suppl):2002
Gilbert M, Dignam J, Won M, Blumenthal D, Vogelbaum MA, Aldape K, Colman H, Chakravarti A, Jeraj R, Armstrong T, Scott Wefel J, Brown P, Jaeckle K, Schiff D, Atkins J, Brachman DG, Werner-Wasik M, Komaki R, Sulman E, Mehta M (2013) RTOG 0825: phase III double-blind placebo-controlled trial evaluating bevacizumab (Bev) in patients (Pts) with newly diagnosed glioblastoma (GBM). J Clin Oncol 31(18 suppl 1):1
Stupp R, Hegi M, Gorlia T, Erridge S, Grujicic D, Steinbach J, Wick W, Tarnawski R, Nam D, Weyerbrock A, Hau P, Taphoorn M, Nabors L, Reardon D, Van Den Bent M, Perry J, Hong Y, Hicking C, Picard M, Weller M (2013) Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation: Final results of the multicenter, randomized, open-label, controlled, phase III CENTRIC study. J Clin Oncol 31(15 suppl LBA2009)
Conflict of interest
The following authors disclose financial relationship with Merck KGaA (Darmstadt, Germany): Bart Neyns (research funding), Michael Weller (research funding and advisory role), Paul M Clément (payment for invited lectures and consultancy), Roger Stupp (consultancy), Jörg Tonn (advisory role and honoraria), Martin Picard (employment). Günter Eisele, Antje Wick, Anna-Carina Eisele, Ghazaleh Tabatabai, Dietmar Krex, Matthias Simon, Uwe Schlegel, Adrian Ochsenbein, Guido Nikkhah and Wolfgang Wick have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Günter Eisele and Antje Wick contributed equally to this work.
Rights and permissions
About this article
Cite this article
Eisele, G., Wick, A., Eisele, AC. et al. Cilengitide treatment of newly diagnosed glioblastoma patients does not alter patterns of progression. J Neurooncol 117, 141–145 (2014). https://doi.org/10.1007/s11060-014-1365-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1365-x