Abstract
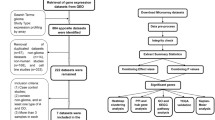
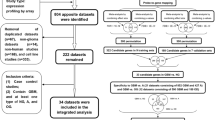
Glioblastoma multiforme (GBM) is the most malignant glioma. In the current study, 149 astrocytoma gene expression datasets were classified by prediction analysis of microarray. Strikingly, disks large homolog 3 (DLG3), a membrane-associated guanylate kinase-family gene, had the highest score in the GBM subset. DLG3 mRNA expression is significantly down-regulated in GBM relative to normal tissue and grade II or grade III astrocytoma according to the results of real-time polymerase chain reaction, and its protein expression shows an obvious difference by immunohistochemistry. Further assays show that DLG3 over-expression induces mitotic cell cycle arrest and apoptosis, and it inhibits proliferation and migration. However, DLG3 over-expression has almost no affect on invasion. The DLG3 protein expression in human brain GBM tissue and its effects on GBM cell invasion were not expected. Our data suggest that DLG3 is down-regulated in this cancer type. To our knowledge, this is the first report to clearly demonstrate the possible involvement of DLG3 in GBM.




Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Weller M (2011) Novel diagnostic and therapeutic approaches to malignant glioma. Swiss Med Wkl 141:w13210
Ohgaki H, Kleihues P (2009) Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci 100(12):2235–2241
Louis DN (2006) Moleculr pathology of malignant gliomas. Annu Rev Pathol 1:97–117
Maher EA, Furnari FB, Bachoo RM et al (2001) Malignant glioma: genetics and biology of a grave matter. Genes Dev 15(11):1311–1333
Zhu Y, Parada LF (2002) The molecular and genetic basis of neurological tumours. Nat Rev Cancer 2:616–626
Ohgaki H, Kleihues P (2005) Epidemiology and etiology of gliomas. Acta Neuropathol 109:93–108
Ohgaki H, Kleihues P (2007) Genetic pathways to primary and secondary glioblastoma. Am J Pathol 170:1445–1453
Lim SK, Llaguno SR, Mckay RM et al (2011) Glioblastoma multiforme: a perspective on recent findings in human cancer and mouse models. BMB rep 44(3):158–164
Sun L, Hui AM, Su Q et al (2006) Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 9(4):287–300
Tibshirani R, Hastie T, Narasimhan B et al (2002) Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci USA 99(10):6567–6572
Sans N, Prybylowski K, Petralia RS et al (2003) NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat Cell Biol 5(6):520–530
Makino K, Kuwahara H, Masuko N et al (1997) Cloning and characterization of NE-dlg: a novel human homolog of the Drosophila discs large (dlg) tumor suppressor protein interacts with the APC protein. Oncogene 14:2425–2433
Norihisa H, Keishi M, Hisashi K et al (2000) NE-dlg, a mammalian homolog of drosophila DLG tumor suppressor, induces growth suppression and impairment of cell adhesion: possible involvement of downregulation of beta-catenin by NE-dlg expression. Int J Cancer 86:480–488
Hiroshi F, Mari M, Mika Y et al (2003) Association of a lung tumor suppressor TSLC1 with MPP3, a human homologue of Drosophila tumor suppressor Dlg. Oncogene 22(40):6160–6165
Ponten J, Macintyre EH (1968) Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand 74(4):465–486
Liu Z, Yao Z, Li Y et al (2011) Gene expression profiling in human high-grade astrocytomas. Comp Funct Genomics. doi:10.1155/2011/245137
Gentleman RC, Carey VJ, Bates DM et al (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5(10):R80
McClintickand JN, Edenberg HJ (2006) Effects of filtering by present call on analysis of microarray experiments. BMC Bioinform 7:49
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 25(4):402–408
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Liu lx, Liu zh, Jiang hc et al (2002) Profiling of differentially expressed genes in human gastric carcinoma by cDNA expression array. World J Gastroenterol 8(4):580–585
Hanada N, Makino K, Koga H et al (2000) NE-dlg, a mammalian homolog of Drosophila dlg tumor suppressor, induces growth suppression and impairment of cell adhesion: possible involvement of down-regulation of β-catenin by NE-dlg expression. Int J Cancer 86(4):480–488
Ishidate T, Matsumine A, Toyoshima K et al (2000) The APC-hDLG complex negatively regulates cell cycle progression from the G0/G1 to S phase. Oncogene 19(3):365–372
Hering H, Sheng M (2002) Direct interaction of Frizzled-1, -2, -4, and -7 with PDZ domains of PSD-95. FEBS Lett 521(1–3):185–189
Karim RZ, Tse G, Putti T et al (2004) The significance of the Wnt pathway in the pathology of human cancers. Pathology 36(2):120–128
Matsumine A, Ogai A, Senda T et al (1996) Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science 272(5264):1020–1023
Chambers AF, Groom AC, MacDonald IC et al (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2:563–572
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3(5):362–374
Bryant PJ, Schmidt O (1990) The genetic control of cell proliferation in Drosophila imaginal discs. J Cell Sci Suppl 13:169–189
Woods DF, Bryant PJ (1991) The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell 66(3):451–464
Ohshiro T, Yagami T, Zhang C et al (2000) Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature 408(6812):593–596
Bellaiche Y, Radovic A, Woods DF et al (2001) The partner of inscuteable/discs-large complex is required to establish planar polarity during asymmetric cell division in Drosophila. Cell 106:355–366
Songyang Z, Fanning AS, Fu C et al (1997) Recognition of unique carboxyl-terminal motifs by distinct pdz domains. Science 275(5296):73–77
Woods DF, Bryant PJ (1989) Molecular cloning of the lethal(1)discs large-1 oncogene of Drosophila. Dev Biol 134(1):222–235
Oliva C, Escobedo P, Astorga C et al (2012) Role of the MAGUK protein family in synapse formation and function. Dev Neurobiol 72(1):57–72
Van Campenhout CA, Eitelhuber A, Gloeckner CJ et al (2011) DLG3 trafficking and apical tight junction formation is regulated by Nedd4 and Nedd4-2 E3 ubiquitin ligases. Dev Cell 21(3):479–491
Thomas U, Phannavong B, Muller B et al (1997) Functional expression of rat synapse-associated proteins SAP97 and SAP102 in Drosophila dlg-1 mutants: effects on tumor suppression and synaptic bouton structure. Mech Dev 62(2):161–174
Cuthbert PC, Stanford LE, Coba MP et al (2007) Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J Neurosci 27(10):2673–2682
Tarpey P, Parnau J, Blow M et al (2004) Mutations in the DLG3 gene cause nonsyndromic X-linked mental retardation. Am J Hum Genet 75(2):318–324
Feldkamp MM et al (1999) Expression of activated epidermal growth factor receptors, Ras-guanosine triphosphate, and mitogen-activated protein kinase in human glioblastoma multiforme specimens. Neurosurgery 45:1442–1453
Nobusawa S et al (2009) IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res 15:6002–6007
Gottfried ON et al (2006) Molecular, genetic, and cellular pathogenesis of neurofibromas and surgical implications. Neurosurgery 58:1–16
Acknowledgments
The authors wish to express their sincere appreciation to Dr. Yangkun Wang and all of the pathologists in the department of pathology at the No. 150 Central Hospital of PLA. We thank them for their support in the specimen pathological diagnosis and grading. Special thanks are due to the reviewers, whose critical eye and enlightened mentoring were instrumental and inspiring.
Conflict of interests
All of the authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhongyu Liu and Yulong Niu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Array intensity distributions (a) and outlier detection for boxplots (b). a Graph A shows boxplots representing the summaries of the signal intensity distributions of the arrays. Each box corresponds to one array. Typically, one expects the boxes to have similar positions and widths. If the distribution of an array is very different from the others, this may indicate an experimental problem. Outlier detection was performed by computing the Kolmogorov-Smirnov statistic Ka between each array’s distribution and the distribution of the pooled data. b Graph B shows a bar chart of the Kolmogorov-Smirnov statistic Ka, and the outlier detection criterion from the previous figure. The bars are shown in the original order of the arrays. Based on the distribution of the values across all arrays, a threshold of 0.319 was determined, which is indicated by the vertical line. None of the arrays exceeded the threshold; therefore, none was considered to be an outlier. Supplementary material 1 (PNG 1641 kb)
Supplementary Fig. 2
Immunohistochemical validation of DLG3 down-regulation in astrocytomas. A section from normal brain tissue (a), from DA tissue (b) from AA tissue (c) and from GBM tissue for staining (d). The staining decreases from normal to GBM.. Supplementary material 2 (JPEG 3720 kb)
Rights and permissions
About this article
Cite this article
Liu, Z., Niu, Y., Xie, M. et al. Gene expression profiling analysis reveals that DLG3 is down-regulated in glioblastoma. J Neurooncol 116, 465–476 (2014). https://doi.org/10.1007/s11060-013-1325-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1325-x