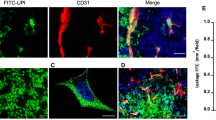
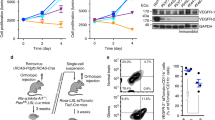
Abstract
The crucial role of angiogenesis in malignant glioma progression makes it a potential target of therapeutic intervention in glioma. Previous studies from our lab showed that sheep erythrocyte membrane glycopeptide T11-target structure (T11TS) has potent anti-neoplastic and immune stimulatory effects in rodent glioma model. In the present study we investigated the anti-angiogenic potential of T11TS and deciphered the underlying molecular mechanism of its anti-angiogenic action in malignant glioma. Vascular endothelial growth factor (VEGF) signaling is crucial for initiating tumor angiogenic responses. The present preclinical study was designed to evaluate the effect of T11TS therapy on VEGF and VEGFR-2 expression in glioma associated brain endothelial cells and to determine the effects of in vivo T11TS administration on expression of PTEN and downstream pro-survival PI3K/Akt/eNOS pathway proteins in glioma associated brain endothelial cells. T11TS therapy in rodent glioma model significantly downregulated expression of VEGF along with its receptor VEGFR-2 and inhibited the expression of pro-survival PI3K/Akt/eNOS proteins in glioma associated brain endothelial cells. Furthermore, T11TS therapy in glioma induced rats significantly upregulated brain endothelial cell PTEN expression, inhibited eNOS phosphorylation and production of nitric oxide in glioma associated brain endothelial cells. Taken together our findings suggest that T11TS can be introduced as an effective angiogenesis inhibitor in human glioma as T11TS targets multiple levels of angiogenic signaling cascade impeding glioma neovascularisation.





Similar content being viewed by others
References
Wen PY, Kesari S (2008) Malignant gliomas in adults. New Eng J Med 359(5):492–507
Plate KH, Breier G, Risau W (1994) Molecular mechanisms of developmental and tumor angiogenesis. Brain Pathol 4(3):207–218
Plate KH (1999) Mechanisms of angiogenesis in the brain. J Neuropathol Exp Neurol 58(4):313–320
Ullrich A (1994) Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature 367(6463):576–579
Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D (2005) Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol 15(4):297–310
Peddinti R et al (2007) Prominent microvascular proliferation in clinically aggressive neuroblastoma. Clin Cancer Res 13(12):3499–3506
Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N (1998) Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273(46):30336–30343
Thakker GD, Hajjar DP, Muller WA, Rosengart TK (1999) The role of phosphatidylinositol 3-kinase in vascular endothelial growth factor signaling. J Biol Chem 274(15):10002–10007
Jiang BH, Zheng JZ, Aoki M, Vogt PK (2000) Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA 97(4):1749–1753
Wen S, Stolarov J, Myers MP, Su JD, Wigler MH, Tonks NK, Durden DL (2001) PTEN controls tumor-induced angiogenesis. Proc Natl Acad Sci USA 98:4622–4627
Giri D, Ittmann M (1999) Inactivation of PTEN tumor suppressor gene is associated with increased angiogenesis in clinically localized prostate carcinoma. Hum Pathol 30(4):419–424
Pan JW, Zhan RY, Tong Y, Zhou YQ, Zhang M (2005) Expression of endothelial nitric oxide synthase and vascular endothelial growth factor in association with neovascularization in human primary astrocytoma. J Zhejiang Univ Sci B 6(7):693–698
Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM (1999) Activation of nitric oxide synthase in endothelial cells by Akt dependent phosphorylation. Nature 399:601–605
Gressett SM, Shah SR (2009) Intricacies of bevacizumab induced toxicities and their management. Ann Pharmacother 43(3):490–501
Ananthnarayan S, Bahng J, Roring J, Nghiemphu P, Lai A, Cloughesy T, Pope WB (2008) Time course of imaging changes of GBM during extended bevacizumab treatment. J Neurooncol 88(3):339–347
Mukherjee J, Ghosh A, Sarkar P, Mazumdar M, Banerjee C, Chaudhuri S (2005) Immunotherapy with T11TS/SLFA-3 specifically induces apoptosis of brain tumor cells by augmenting intracranial immune status. Anticancer Res 25(4):2905–2919
Acharya S, Chatterjee S, Kumar P, Bhattacharjee M, Chaudhuri S, Chaudhuri S (2009) Induction of G1 arrest in glioma cells by T11TS is associated with upregulation of Cip1/Kip1 and concurrent downregulation of cyclin D (1 and 3). Anticancer Drugs 21(1):53–64
Bhattacharjee M, Acharya S, Ghosh A, Sarkar P, Chatterjee S, Kumar P, Chaudhuri S (2008) Bax and Bid act in synergy to bring about T11TS mediated glioma apoptosis via the release of mitochondrial cytochrome c and subsequent caspase activation. Int Immunol 20(12):1489–1505
Kumar P, Acharya S, Chatterjee S, Kumari A, Chaudhuri S, Singh MK, Ghosh SN, Chaudhuri S (2012) Immunomodulatory role of TIITS in respect to cytotoxic lymphocytes in four grades of human glioma. Cell Immunol 276(1–2):176–186
Hunig T, Mitnacht R, Tiefenthaler G, Kohler C, Miyasaka M (1986) T11TS, the cell surface molecule binding to the “erythrocyte receptor” of T lymphocytes: cellular distribution, purification to homogeneity and biochemical properties. Eur J Immunol 16(12):1615–1621
Hunig T (1985) The cell surface molecule recognized by the erythrocyte receptor of T lymphocyte: identification and partial characterization using a monoclonal antibody. J Exp Med 162:890–901
Sarkar P, Bhattacharjee M, Acharya S, Ghosh A, Tripathi SK, Chaudhuri S (2007) Acute toxicity studies of T11TS: a glycopeptide with antineoplastic effects against glioma. Toxicol Int 14(1):47–56
Sarkar P, Bhattcharjee M, Acharya S, Das Gupta S, Guha D, Sadhu M, Chaudhuri S (2010) Subacute toxicity study of T11TS, a novel immunotherapeutic glycopeptide against glioma. Adv Pharmacol Toxicol 11(1):1–10
Mukherjee J, Sarkar S, Begum Z, Dutta S, Ghosh A, Chaudhuri S, Chaudhuri S (2002) Preclinical Changes in immunoreactivity and cellular architecture during the progressive development of intracranial neoplasms and an immunotherapeutic schedule with a novel biological response modifier, the T11TS/S-LFA3 Asian Pac J. Cancer Prev 3(4):325–337
Mukherjee J, Sarkar S, Ghosh A, Dutta Gupta AK, Chaudhuri S, Chaudhuri S (2004) Immunotherapeutic effects of T11TS/SLFA-3 against nitrosocompound mediated neural genotoxicity. Toxicol Lett 150(3):239–257
Lantos PL (1993) Chemical induction of tumors in nervous system. In: Thomas GT (ed) Neurooncology. Churchill Livingstone, New York, London, pp 85–107
Abbott NJ, Hughes CCW, Revest PA, Greenwood J (1992) Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J Cell Sci 103:23–37
Beijnum JR, Rousch M, Castermans K, Linden E, Griffioen A (2008) Isolation of endothelial cells from fresh tissues. Nat Protoc 3(6):1085–1091
Ding AH, Nathan CF, Stuehr DJ (1988) Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol 141(7):2407–2412
Waltenberger J, Claessonwelsh L, Siegbahn A, Shibuya M, Heldin CH (1994) Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 269:26988–26995
Yoshida A, Anand-Apte B, Zetter BR (1996) Differential endothelial migration and proliferation to basic fibroblast growth factor and vascular endothelial growth factor. Growth Factors 13(1–2):57–64
Nicholson KM, Anderson NG (2002) The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal 14(5):381–395
Onishi M, Ichikawa T, Kurozumi K, Date I (2011) Angiogenesis and invasion in glioma. Brain Tumor Pathol 28:13–24
Siemerink MJ, Klaassen I, Vogels IM, Griffioen AW, Van Noorden CJ, Schlingemann RO (2012) CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis 15(1):151–163
Wang D, Stockard CR, Harkins L, Lott P, Salih C, Yuan K, Buchsbaum D, Hashim A, Zayzafoon M, Hardy RW, Hameed O, Grizzle W, Siegal GP (2008) Immunohistochemistry in the evaluation of neovascularization in tumor xenografts. Biotech Histochem 83(3):179–189
Guangqi E, Cao Y, Bhattacharya S, Dutta S, Wang E, Mukhopadhyay D (2012) Endogenous vascular endothelial growth factor (VEGF): A maintains endothelial cell homeostasis by regulating VEGF receptor-2 transcription. J Biol Chem 287(5):3029–3041
Waters JD, Sanchez C, Sahin A, Futalan D, Gonda DD, Scheer JK, Akers J, Palanichamy K, Waterman P, Chakravarti A, Weissleder R, Morse B, Marsh N, Furfine E, Chen CC, Carvajal I, Carter BS (2012) CT322, a VEGFR-2 antagonist, demonstrates anti-glioma efficacy in orthotopic brain tumor model as a single agent or in combination with temozolomide and radiation therapy. J Neurooncol 110(1):37–48
Fujii M, Inoki I, Saga M, Morikawa N, Arakawa K, Inaba S, Yoshioka K, Konoshita T, Miyamori I (2012) Aldosterone inhibits endothelial morphogenesis and angiogenesis through the downregulation of vascular endothelial growth factor receptor-2 expression subsequent to peroxisome proliferator-activated receptor gamma. J Steroid Biochem Mol Biol 129(3–5):145–152
Meissner M, Doll M, Hrgovic I, Reichenbach G, Konig V, Hailemariam-Jahn T, Gille J, Kaufmann R (2011) Suppression of VEGFR2 expression in human endothelial cells by dimethylfumarate treatment: evidence for anti-angiogenic action. Journal of Investigative Dermatology 131(6):1356–1364
Munaut C, Noel A, Hougrand O, Foidart JM, Boniver J, Manuel Deprez M (2003) Vascular endothelial growth factor expression correlates with matrix metalloproteinases Mt1-MMP, MMP-2 and MMP-9 in human glioblastomas. Int J Cancer 106(6):848–855
Dance M, Montagner A, Yart A, Masri B, Audigier Y, Perret B, Salles JP, Raynal P (2006) The adaptor protein Gab1 couples the stimulation of vascular endothelial growth factor receptor-2 to the activation of phosphoinositide 3-kinase. J Biol Chem 281(32):23285–23295
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-kinase Akt pathway in human cancer. Nat Rev Cancer 2(7):489–501
Ma J, Sawai H, Ochi N, Matsuo Y, Xu D, Yasuda A, Takahashi H, Wakasugi T, Takeyama H (2009) PTEN regulates angiogenesis through PI3 K/Akt/VEGF signaling pathway in human pancreatic cancer cells. Mol Cell Biochem 331(1–2):161–171
Xiao D, Li M, Herman-Antosiewicz A, Antosiewicz J, Xiao H, Lew KL, Zeng Y, Marynowski SW, Singh SV (2006) Diallyl trisulfide inhibits angiogenic features of human umbilical vein endothelial cells by causing Akt inactivation and down-regulation of VEGF and VEGFR-2. Nutr Cancer 55(1):94–107
Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC (1999) Regulation of endothelium derived nitric oxide production by the protein kinase Akt. Nature 399(6736):597–601
Michell BJ, Griffiths JE, Mitchelhill KI, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano PR, Kemp BE, Pearson RB (1999) The Akt kinase and AMP kinases signal directly to endothelial nitric oxide synthase. Curr Biol 9(15):845–848
Kawasaki K, Smith RS Jr, Hsieh C, Sun J, Chao J, Liao JK (2003) Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol 23(16):5726–5737
Urbich C, Reissner A, Chavakis E, Dernbach E, Haendeler J, Fleming I, Zeiher AM, Kaszkin M, Dimmeler S (2002) Dephosphorylation of endothelial nitric oxide synthase contributes to the anti-angiogenic effects of endostatin. FASEB J 16(7):706–708
Hood JD, Meininger CJ, Ziche M, Granger HJ (1998) VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol 274:H1054–H1058
Mukherjee J (2004) Characterization of T11TS/SLFA-3 and its application as an immunomodulatory adjunct in brain tumor therapy: an immunomolecular and genetic approach in animal models. PhD Dissertation, University of Calcutta
Tiefenthaler G, Dustin ML, Springer TA, Hünig T (1987) Serologic cross-reactivity of T11 target structure and lymphocyte function-associated antigen 3. Evidence for structural homology of the sheep and human ligands of CD2. J Immunol 139(8):2696–2701
Acknowledgments
The study was supported by funding from the Department of Science and Technology (DST) Govt. of India. The authors wish to thank Dr. Pijush Kanti Das, Chief Scientist and Head of Infectious Disease and Immunology Division at the CSIR-Indian Institute of Chemical Biology for providing immunofluorescence imaging facilities and some monoclonal antibodies. The authors also wish to thank Ms. Shreyasi Palit, senior research fellow at CSIR-IICB for technical help.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhattacharya, D., Singh, M.K., Chaudhuri, S. et al. T11TS impedes glioma angiogenesis by inhibiting VEGF signaling and pro-survival PI3K/Akt/eNOS pathway with concomitant upregulation of PTEN in brain endothelial cells. J Neurooncol 113, 13–25 (2013). https://doi.org/10.1007/s11060-013-1095-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1095-5