Abstract
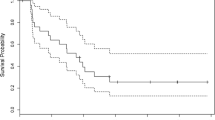
The objective of this prospective, monocentric phase-II pilot study was to evaluate toxicity and efficacy of neoadjuvant temozolomide (TMZ) and 13-cis retinoic acid (13-cRA) treatment in patients with newly diagnosed anaplastic gliomas after total or subtotal tumor resection. The primary endpoint of the study was median progression-free survival (PFS). Secondary endpoints were toxicity and PFS rates at 6, 12 and 24 months. Thirty-two adult patients were included in the study and treated with a median number of 10 TMZ and 13-cRA cycles (range 1–26). The majority of patients had favorable prognostic factors characterized by young age, complete resection, oligodendroglial histology, 1p/19q co-deletion, O6-methylguanine-DNA methyltransferase (MGMT) promotor methylation and isocitrate dehydrogenase 1 (IDH1) mutation. Grade 3/4 myelotoxicity occurred in 5/32 patients, and about 90% of patients suffered from grade 2/3 adverse events attributable to 13-cRA. The median PFS was 37.8 months (95% CI 22.2–53.4). The 6-, 12- and 24-month PFS rates were 84.4, 75 and 42.4%. The extent of tumor resection was the only prognostic factor associated with better PFS. TMZ and 13-cRA treatment did not improve PFS when retrospectively compared to the TMZ-treated group within the randomized NOA-04 phase-III trial. In conclusion, 13-cRA addition to TMZ in a neoadjuvant setting showed acceptable toxicity, but did not yield an advantage in PFS in patients with newly diagnosed anaplastic gliomas after total or subtotal tumor resection.

Similar content being viewed by others
References
Bouterfa H, Picht T, Kess D, Herbold C, Noll E, Black PM, Roosen K, Tonn JC (2000) Retinoids inhibit human glioma cell proliferation and migration in primary cell cultures but not in established cell lines. Neurosurgery 46:419–430
Costa SL, Paillaud E, Fages C, Rochette-Egly C, Plassat JL, Jouault H, Perzelova A, Tardy M (2001) Effects of a novel synthetic retinoid on malignant glioma in vitro: inhibition of cell proliferation, induction of apoptosis and differentiation. Eur J Cancer 37:520–530
Paillaud E, Costa S, Fages C, Plassat JL, Rochette-Egly C, Monville C, Tardy M (2002) Retinoic acid increases proliferation rate of GL-15 glioma cells, involving activation of STAT-3 transcription factor. J Neurosci Res 67:670–679
Das A, Banik NL, Ray SK (2008) Retinoids induced astrocytic differentiation with down regulation of telomerase activity and enhanced sensitivity to taxol for apoptosis in human glioblastoma T98G and U87MG cells. J Neurooncol 87:9–22
Campos B, Wan F, Farhadi M, Ernst A, Zeppernick F, Tagscherer KE, Ahmadi R, Lohr J, Dictus C, Gdynia G, Combs SE, Goidts V, Helmke BM, Eckstein V, Roth W, Beckhove P, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende C (2010) Differentiation therapy exerts antitumor effects on stem-like glioma cells. Clin Cancer Res 16:2715–2728
Meyskens FL Jr., Goodman GE, Alberts DS (1985) 13-cis-Retinoic acid: pharmacology, toxicology, and clinical applications for the prevention and treatment of human cancer. Crit Rev Oncol Hematol 3:75–101
Le Doze F, Debruyne D, Albessard F, Barre L, Defer GL (2000) Pharmacokinetics of all-trans retinoic acid, 13-cis retinoic acid, and fenretinide in plasma and brain of Rat. Drug Metab Dispos 28:205–208
Yung WK, Kyritsis AP, Gleason MJ, Levin VA (1996) Treatment of recurrent malignant gliomas with high-dose 13-cis-retinoic acid. Clin Cancer Res 2:1931–1935
Wismeth C, Hau P, Fabel K, Baumgart U, Hirschmann B, Koch H, Jauch T, Grauer O, Drechsel L, Brawanski A, Bogdahn U, Steinbrecher A (2004) Maintenance therapy with 13-cis retinoid acid in high-grade glioma at complete response after first-line multimodal therapy—a phase-II study. J Neurooncol 68:79–86
Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O’Neill AM, Friedman AH, Bruner J, Yue N, Dugan M, Zaknoen S, Levin VA (1999) Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol 17:2762–2771
Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W, Glantz M, Greenberg H, Selker RG, Vick NA, Rampling R, Friedman H, Phillips P, Bruner J, Yue N, Osoba D, Zaknoen S, Levin VA (2000) A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 83:588–593
Chibbaro S, Benvenuti L, Caprio A, Carnesecchi S, Pulera F, Faggionato F, Serino D, Galli C, Andreuccetti M, Buxton N, Gagliardi R (2004) Temozolomide as first-line agent in treating high-grade gliomas: phase II study. J Neurooncol 67:77–81
Reid JM, Stevens DC, Rubin J, Ames MM (1997) Pharmacokinetics of 3-methyl-(triazen-1-yl)imidazole-4-carboximide following administration of temozolomide to patients with advanced cancer. Clin Cancer Res 3:2393–2398
Ostermann S, Csajka C, Buclin T, Leyvraz S, Lejeune F, Decosterd LA, Stupp R (2004) Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res 10:3728–3736
Stupp R, Dietrich PY, Ostermann Kraljevic S, Pica A, Maillard I, Maeder P, Meuli R, Janzer R, Pizzolato G, Miralbell R, Porchet F, Regli L, de Tribolet N, Mirimanoff RO, Leyvraz S (2002) Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol 20:1375–1382
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Jaeckle KA, Hess KR, Yung WK, Greenberg H, Fine H, Schiff D, Pollack IF, Kuhn J, Fink K, Mehta M, Cloughesy T, Nicholas MK, Chang S, Prados M (2003) Phase II evaluation of temozolomide and 13-cis-retinoic acid for the treatment of recurrent and progressive malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol 21:2305–2311
Butowski N, Prados MD, Lamborn KR, Larson DA, Sneed PK, Wara WM, Malec M, Rabbitt J, Page M, Chang SM (2005) A phase II study of concurrent temozolomide and cis-retinoic acid with radiation for adult patients with newly diagnosed supratentorial glioblastoma. Int J Radiat Oncol Biol Phys 61:1454–1459
Corn BW, Yousem DM, Scott CB, Rotman M, Asbell SO, Nelson DF, Martin L, Curran WJ Jr. (1994) White matter changes are correlated significantly with radiation dose. Observations from a randomized dose-escalation trial for malignant glioma (Radiation Therapy Oncology Group 83–02). Cancer 74:2828–2835
Crossen JR, Garwood D, Glatstein E, Neuwelt EA (1994) Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol 12:627–642
Gilbert MR, Friedman HS, Kuttesch JF, Prados MD, Olson JJ, Reaman GH, Zaknoen SL (2002) A phase II study of temozolomide in patients with newly diagnosed supratentorial malignant glioma before radiation therapy. Neuro Oncol 4:261–267
Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, Sabel MC, Koeppen S, Ketter R, Meyermann R, Rapp M, Meisner C, Kortmann RD, Pietsch T, Wiestler OD, Ernemann U, Bamberg M, Reifenberger G, von Deimling A, Weller M (2009) NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol 27:5874–5880
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol (Berl) 114:97–109
Macdonald DR, Cascino TL, Schold SC Jr., Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG (1999) Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res 59:793–797
Mueller W, Mizoguchi M, Silen E, D’Amore K, Nutt CL, Louis DN (2005) Mutations of the PIK3CA gene are rare in human glioblastoma. Acta Neuropathol 109:654–655
Jeuken J, Cornelissen S, Boots-Sprenger S, Gijsen S, Wesseling P (2006) Multiplex ligation-dependent probe amplification: a diagnostic tool for simultaneous identification of different genetic markers in glial tumors. J Mol Diagn 8:433–443
Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A (2008) Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol 116:597–602
van den Bent MJ, Dubbink HJ, Sanson M, van der Lee-Haarloo CR, Hegi M, Jeuken JW, Ibdaih A, Brandes AA, Taphoorn MJ, Frenay M, Lacombe D, Gorlia T, Dinjens WN, Kros JM (2009) MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors: a report from EORTC Brain Tumor Group Study 26951. J Clin Oncol 27:5881–5886
Brandes AA, Tosoni A, Cavallo G, Reni M, Franceschi E, Bonaldi L, Bertorelle R, Gardiman M, Ghimenton C, Iuzzolino P, Pession A, Blatt V, Ermani M (2006) Correlations between O6-methylguanine DNA methyltransferase promoter methylation status, 1p and 19q deletions, and response to temozolomide in anaplastic and recurrent oligodendroglioma: a prospective GICNO study. J Clin Oncol 24:4746–4753
Kouwenhoven MC, Kros JM, French PJ, Biemond-ter Stege EM, Graveland WJ, Taphoorn MJ, Brandes AA, van den Bent MJ (2006) 1p/19q loss within oligodendroglioma is predictive for response to first line temozolomide but not to salvage treatment. Eur J Cancer 42:2499–2503
Griffin CA, Burger P, Morsberger L, Yonescu R, Swierczynski S, Weingart JD, Murphy KM (2006) Identification of der(1;19)(q10;p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J Neuropathol Exp Neurol 65:988–994
Jenkins RB, Blair H, Ballman KV, Giannini C, Arusell RM, Law M, Flynn H, Passe S, Felten S, Brown PD, Shaw EG, Buckner JC (2006) A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res 66:9852–9861
Ichimura K, Vogazianou AP, Liu L, Pearson DM, Backlund LM, Plant K, Baird K, Langford CF, Gregory SG, Collins VP (2008) 1p36 is a preferential target of chromosome 1 deletions in astrocytic tumours and homozygously deleted in a subset of glioblastomas. Oncogene 27:2097–2108
Idbaih A, Marie Y, Pierron G, Brennetot C, Hoang-Xuan K, Kujas M, Mokhtari K, Sanson M, Lejeune J, Aurias A, Delattre O, Delattre JY (2005) Two types of chromosome 1p losses with opposite significance in gliomas. Ann Neurol 58:483–487
Hartmann C, Johnk L, Kitange G, Wu Y, Ashworth LK, Jenkins RB, Louis DN (2002) Transcript map of the 3.7-Mb D19S112–D19S246 candidate tumor suppressor region on the long arm of chromosome 19. Cancer Res 62:4100–4108
Hartmann C, Mueller W, Lass U, Kamel-Reid S, von Deimling A (2005) Molecular genetic analysis of oligodendroglial tumors. J Neuropathol Exp Neurol 64:10–14
Gan HK, Rosenthal MA, Dowling A, Kalnins R, Algar E, Wong N, Benson A, Woods A-M, Cher L (2010) A phase II trial of primary temozolomide in patients with grade III oligodendroglial brain tumors. Neuro Oncol 12:500–507
Acknowledgments
We cordially thank Birgit Hirschmann and Christiane Reinert for compiling the data for this analysis. We further thank Christel Herold-Mende and Benito Campos from the Department of Neurosurgery, University of Heidelberg, for analysing the retinoic acid signalling pathway expression in our dataset.
Funding
The study was supported by Essex Pharma (Schering-Plough Germany) with an unrestricted educational grant.
Conflict of interest
Ulrich Bogdahn: travel support by Schering-Plough; scientific advisor and speaker for Schering-Plough. Peter Hau: travel support by Schering-Plough; scientific advisor and speaker for Schering-Plough; several unrestricted educational grants by Schering-Plough. Wolfgang Wick: travel support by Schering-Plough; scientific advisor and speaker for Schering-Plough. Michael Weller: travel support by Schering-Plough; scientific advisor and speaker for Schering-Plough.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s11060-011-0742-y
Rights and permissions
About this article
Cite this article
Grauer, O., Pascher, C., Hartmann, C. et al. Temozolomide and 13-cis retinoic acid in patients with anaplastic gliomas: a prospective single-arm monocentric phase-II study (RNOP-05). J Neurooncol 104, 801–809 (2011). https://doi.org/10.1007/s11060-011-0548-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0548-y