Abstract
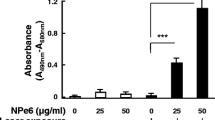
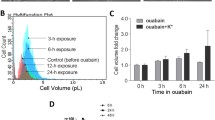
The basic mechanism of cell death induced by 5-aminolevulinic acid (5-ALA)-mediated photodynamic therapy (PDT) (ALA-PDT) in glioma cells has not been fully elucidated. In this study, the details of the cell death mechanism induced by ALA-PDT were investigated in three human glioma cell lines (U251MG, U87MG, and U118MG) in vitro. To evaluate the manner of accumulation of protoporphyrin IX (PpIX), intracellular PpIX contents were measured by flow cytometry after incubation with 5-ALA. To analyze the mechanism of cell death, U251MG cells were assayed by the terminal deoxynucleotidyl transferase-mediated dUTP-FITC nick end-labeling (TUNEL) method, and the caspase activity was measured after ALA-PDT. Furthermore, the mitochondrial membrane potential (MMP) and the release of mitochondrial cytochrome c were determined. PpIX fluorescence reached a plateau 4 h after exposure to 5-ALA. The proportion of dead cells increased with increases in the dosage of light. These cells were confirmed by TUNEL staining to be apoptotic. Increases in the activity of both caspase-3 and -9, a decrease in MMP, and a marked increase in cytochrome c in the cytosolic fraction were found after cells were subjected to PDT. These results indicate that a dysfunction of MMP is followed by mitochondrial cytochrome c release, which triggers apoptosis through a mitochondrial pathway. ALA-PDT induces massive apoptosis due to the direct activation of a mitochondrial pathway, which is resistant to many anti-apoptotic processes, in human glioma cells. This finding implies that ALA-PDT is a promising therapy for the treatment of apoptosis-reluctant tumors such as malignant gliomas.








Similar content being viewed by others
Abbreviations
- 5-ALA:
-
5-aminolevulinic acid
- MMP:
-
mitochondrial membrane potential
- PDT:
-
photodynamic therapy
- PpIX:
-
protoporphyrin IX
- TUNEL:
-
terminal deoxynucleotidyl transferase-mediated dUTP-FITC nick end-labeling
References
Agarwal ML, Clay ME, Harvey EJ, Antunez AR, Oleinick NL (1991) Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells. Cancer Res 51:5993–5996
Carthy CM, Granville DJ, Jiang H, Levy J, Rudin CM, Thompson CB, McManus BM, Hunt DWC (1999) Early release of mitochondrial cytochrome c and expression of mitochondrial epitope 7A6 with a porphyrin-derived photosensitizer: Bcl-2 and Bcl-xL overexpression do not prevent early mitochondrial events but still depress caspase activity. Lab Invest 79:953–965
Casciola-Rosen L, Nicholson DW, Chong T, Rowan KR, Thornberry NA, Miller DK, Rosen A (1996) Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J Exp Med 183:1957–1964
Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q (1998) Photodynamic therapy. J Natl Cancer I 90:889–900
El-Sharabasy MMH, El-Waseef AM, Hafez MM, Salim SA (1992) Porphyrin metabolism in some malignant disease. Br J Canc 65:409–412
Gibson SL, Cupriks DL, Havens JJ, Nguyen ML (1998) A regulatory role for porphobilinogen deaminase (PBGD) in δ-aminolevulinic acid (δ-ALA)-induced photosensitization. Br J Canc 77:235–243
Gomez-Manzano C, Fueyo J, Kyritsis AP, Steck PA, Roth JA, McDonnell TJ, Steck KD, Levin VA, Yung WK (1996) Adenovirus-mediated transfer of the p53 gene produces rapid and generalized death of human glioma cells via apoptosis. Cancer Res 56:694–699
Gossner L, May A, Sroka R, Stolte M, Hahn EG, Ell C (1999) Photodynamic destruction of high grade dysplasia and early carcinoma of the esophagus after the oral administration of 5-aminolevulinic acid. Cancer 15:1921–1928
Gossner L, Stolte M, Sroka R, Rick K, May A, Hahn EG, Ell C (1998) Photodynamic ablation of high-grade dysplasia and early cancer in Barrett’s esophagus by means of 5-aminolevulinic acid. Gastroenterology 114:448–455
Grant WE, Hopper C, MacRobert AJ, Speight M, Bown SG (1993) Photodynamic therapy of oral cancer: photosensitization with systemic aminolevulinic acid. Lancet 342:147–148
Granville DJ, Carthy CM, Jiang H, Shore GC, McManus BM, Hunt DW (1998) Rapid cytochrome c release, activation of caspases 3, 6, 7 and 8 followed by Bap31 cleavage in HeLa cells treated with photodynamic therapy. FEBS Lett 437:5–10
Grebeňová D, Kuželová K, Smetana K, Pluskalová M, Cajthamlová H, Marinov I, Fuchs O, Souček J, Jarolím P, Hrkal Z (2003) Mitochondrial and endoplasmic reticulum stress-induced apoptotic pathways are activated by 5-aminolevulinic acid-based photodynamic therapy in HL60 leukemia cells. J Photochem Photobiol B 69:71–85
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Henderson BW, Dougherty TJ (1992) How does photodynamic therapy work?. Photochem Photobiol 55:145–157
Hengartner MO (2000) The biochemistry of apoptosis. Nature 407:770–776
Iinuma S, Farshi SS, Ortel B, Hasan T (1994) A mechanistic study of cellular photodestruction with 5-aminolaevulinic acid-induced porphyrin. Br J Cancer 70:21–28
Kaye AH, Hill JS (1992) Photodynamic therapy of cerebral tumors. Neurosurg Q 1:233–258
Kaye AH, Morstyn G, Apuzzo MLJ (1988) Photoradiation therapy and its potential in the management of neurological tumours. J Neurosurg 69:1–14
Kennedy JC, Pottier RH (1992) Endogenous protoporphyrin IX: a clinical useful photosensitizer for photodynamic therapy. J Photochem Photobiol B 14:275–292
Kessel D, Luo Y (1999) Photodynamic therapy: a mitochondrial inducer of apoptosis. Cell Death Differ 6:28–35
Kostron H, Obwegesser A, Jacober R (1996) Photodynamic therapy in neurosurgery: a review. J Photochem Photobiol B 36:157–168
Kroemer G, Zamzami N, Susin SA (1997) Mitochondrial control of apoptosis. Immunol Today 18:44–51
Li P, Nijhawan D, Budihardjo I, Srinivasla SM, Ahmad M, Alnemri ES, Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489
Lilge L, Wilson BC (1998) Photodynamic therapy of intracranial tissues: a preclinical comparative study of four different photosensitizers. J Clin Laser Med Surg 16:81–92
Luo Y, Chang CK, Kessel D (1996) Rapid initiation of apoptosis by photodynamic therapy. Photochem Photobiol 63:528–534
Martins LM, Earnshaw WC (1997) Apoptosis: alive and kicking in 1997. Trends Cell Biol 7:111–114
Muller PJ, Wilson BC (1995) Photodynamic therapy for recurrent supratentorial gliomas. Semin Surg Oncol 11:346–354
Nicholson DW, Thornberry NA (1997) Caspases: killer proteases. Trends Biochem Sci 22:299–306
Oleinick NL, Evans HH (1998) The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res 150:146–156
Olzowy B, Hundt CS, Stocker S, Bise K, Reulen HJ, Stummer W (2002) Photoirradiation therapy of experimental malignant glioma with 5-aminolevulinic acid. J Neurosurg 97:970–976
Peng Q, Berg K, Moan J, Kongshaug M, Nesland JM (1997) 5-Aminolevulinic acid-based photodynamic therapy: principles and experimental research. Photochem Photobiol 65:235–251
Peng Q, Warloe T, Berg K, Moan J, Kongshaug M, Giercksky K-E, Nesland JM (1997) 5-aminolevulinic acidbased photodynamic therapy: clinical research and future challenges. Cancer 79:2282–2308
Regula J, MacRobert AJ, Gorchein A, Buonaccorsi GA, Thorpe SM, Spencer GM, Hatfield AR, Bown SG (1995) Photosensitization and photodynamic therapy of oesophageal, duodenal and colorectal tumors using 5-aminolevulinic acid induced protoporphyrin IX—a pilot study. Gut 36:67–75
Rosenthal MA, Kavar B, Hill JS, Morgan DJ, Nation RL, Stylli SS, Basser RL, Uren S, Geldard H, Green MD, Kahl SB, Kaye AH (2001) Phase I and pharmacokinetic study of photodynamic therapy for high-grade gliomas using a novel boronated porphyrin. J Clin Oncol 19:519–524
Schlegel J, Peters I, Orrenius S, Miller DK, Thornberry NA, Yamin TT, Nicholson DW (1996) CPP32/Apopain is a key interleukin 1b converting enzyme-like protease involved in Fas-mediated apoptosis. J Biol Chem 271:1841–1844
Shapiro WR, Green SB, Burger PC et al (1989) Randomized trial of three chemotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Group Trial 8001. J Neurosurg 71:1–9
Shibata M, Horiguchi T, Morimoto J, Otsuki Y (2003) Massive apoptotic cell death in chemically induced rat urinary bladder carcinomas following in situ HSVtk electrogene transfer. J Gene Med 5:219–231
Stummer W, Novotny A, Stepp H et al (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93:1003–1013
Stummer W, Stocker S, Novotny A et al (1998) In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J Photochem Photobiol B 45:160–169
Stummer W, Stocker S, Wagner S et al (1998) Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 42:518–526
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275:1129–1132
Zardi SI, Oleinick NL, Zain MT, Mukhtar H (1993) Apoptosis during photodynamic therapy-induced ablative RIF-1 tumors in C3H mice. Photochem Photobiol 58:771–776
Zou H, Li Y, Liu X, Wang X (1999) An APAF-1-cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem 274:11549–11556
Acknowledgment
We would like to thank Teruo Ueno of the Central Research Laboratory at Osaka Medical College for his technical support with the flow cytometric analysis.
Investment/Financial. Disclosure:
This work was supported by Grants-in-Aid for Scientific Research (C) (14571345), (C) (15500351), and by a Grant-in-Aid for Exploratory Research (14657350) from the Japanese Ministry of Education, Science and Culture, awarded to Drs. T. Kuroiwa, Y. Kajimoto, and S. Miyatake, respectively. Additional support was provided in the form of a grant from the Promotion and Mutual Aid Corporation for Private Schools of Japan and the Science Research Promotion Fund to Dr. S. Miyatake and by a grant from the High-Tech Research Program of Osaka Medical College.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inoue, H., Kajimoto, Y., Shibata, MA. et al. Massive apoptotic cell death of human glioma cells via a mitochondrial pathway following 5-aminolevulinic acid-mediated photodynamic therapy. J Neurooncol 83, 223–231 (2007). https://doi.org/10.1007/s11060-006-9325-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-006-9325-8