Abstract
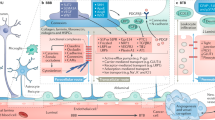
Although knowledge of molecular biology and cellular physiology has advanced at a rapid pace, much remains to be learned about delivering chemotherapy and antibodies across the blood–brain barrier (BBB) for the diagnosis and treatment of central nervous system (CNS) disease. A meeting, partially funded by an NIH R13 grant, was convened to discuss the state of the science, current knowledge gaps, and future directions in the delivery of drugs and proteins to the CNS, for the treatment of primary and metastatic brain tumors. Meeting topics included CNS metastases and the BBB, and chemoprotection and chemoenhancement in CNS disorders. The discussions regarding CNS metastases generated possibilities of chemoprotection as a means not only to decrease treatment-related toxicity but also to increase chemotherapy dose intensity. The increasing incidence of sanctuary brain metastasis from breast cancer, in part due to the difficulty of monoclonal antibodies (mAbs) such as herceptin to cross the BBB, was one of the most salient “take home” messages of the meeting.
Similar content being viewed by others
References
Lin NU, Bellon JR, Winer EP (2004) CNS metastases in breast cancer. J Clin Oncol 22:3608–3617
Nathoo N, Chahlavi A, Barnett GH, Toms SA (2005) Pathobiology of brain metastases. J Clin Pathol 58:237–242
Report of the Brain Tumor Progress Review Group (November 2000) National Cancer Institute, National Institute of Neurological Disorders and Stroke
Walsh JW (1996) Biology of a brain metastasis. Neurosurg Clin N Am 7:369–376
Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 1:571–573
Kirsch M, Schackert G, Black PM (2004) Metastasis and angiogenesis. Cancer Treat Res 117:285–304
Khanna C, Hunter K (2005) Modeling metastasis in vivo. Carcinogenesis 26:513–523
Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD (2002) The seed and soil hypothesis: vascularization and brain metastases. Lancet Oncol 3:53–57
Myklebust AT, Helseth A, Breistol K, Hall WA, Fodstad O (1994) Nude rat models for human tumor metastasis to CNS. Procedures for intracarotid delivery of cancer cells and drugs. J Neurooncol 21:215–224
Rye PD, Norum L, Olsen DR, Garman-Vik S, Kaul S, Fodstad O (1996) Brain metastasis model in athymic nude mice using a novel MUC1-secreting human breast-cancer cell line. Int J Cancer 68:682–687
Weil RJ, Palmieri D, Bronder JL, Stark AM, Steeg PS (2005) Breast cancer metastasis to the central nervous system. Am J Pathol 167:913–920
Howard RB, Mullen JB, Pagura ME, Johnston MR (1999) Characterization of a highly metastatic, orthotopic lung cancer model in the nude rat. Clin Exp Metastasis 17:157–162
Knopp MV, Runge VM, Essig M, Hartman M, Jansen O, Kirchin MA et al (2004) Primary and secondary brain tumors at MR Imaging: bicentric intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine. Radiology 230:55–64
Trattnig S, Ba-Ssalamah A, Noebauer-Huhmann I-M, Barth M, Wolfsberger S, Pinker K et al (2003) MR contrast agent at high-field MRI (3 Tesla). Top Magn Reson Imaging 14:365–375
Moffat BA, Chenevert TL, Lawrence TS, Charles MR, Johnson TD, Dong Q et al (2005) Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci USA 102:5524–5529
Essig M, Waschkies M, Wenz F, Debus J, Hentrich HR, Knopp MV (2003) Assessment of brain metastases with dynamic susceptibility-weighted contrast enhanced MR imaging: initial results. Radiology 228:193–199
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Eng J Med 350:2129–2139
Hegi ME, Diserens A-C, Gorlia T, Hamou M-F, de Tribolet N, Weller M et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Eng J Med 352:997–1003
Bafaloukos D, Tsoutsos D, Fountzilas G, Linardou H, Christodoulou C, Kalofonos H (2004) The effect of temozolomide-based chemotherapy in patients with cerebral metastases from melanoma. Melanoma Res 14:289–294
Grossman SA, Fisher JD, Piantadosi S, Brem H (1998) The new approaches to brain tumor therapy (NABTT) CNS consortium: organization, objectives, and activities. Cancer Control 5:107–114
Chiu C-H, Tsai C-M, Chen Y-M, Chiang S-C, Liou J-L, Perng R-P (2005) Gefitinib is active in patients with brain metastases from non-small cell lung cancer and response is related to skin toxicity. Lung Cancer 47:129–138
Alas S, Ng CP, Bonavida B (2002) Rituximab modifies the cisplatin-mitochondrial signaling pathway, resulting in apoptosis in cisplatin-resistant non-Hodgkins lymphoma. Clin Cancer Res 8:836–845
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792
Pestalozzi BC, Brignoli S (2000) Trastuzumab in CSF. J Clin Oncol 18:2350–2351
Loesch D, Robert N, Asmar L, Gregurich MA, O’Rourke M, Dakhil S et al (2002) Phase II multicenter trial of a weekly paclitaxel and carboplatin regimen in patients with advanced breast cancer. J Clin Oncol 20:3857–3864
Dirnagl U, Iadecola C, Moskowitz MA (1997) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22: 391–397
Wu G, Fang Y-Z, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J␣Nutr 134:489–492
Gilmont RR, Dardano A, Young M, Engle JS, Adamson BS, Smith DJ Jr et al (1998) Effects of glutathione depletion on oxidant-induced endothelial cell injury. J Surg Res 80:62–68
Kim SO, Cho IS, Gu HK, Lee DH, Lim H, Yoo SE (2004) KR-31378 protects neurons from ischemia-reperfusion brain injury by attenuating lipid peroxidation and glutathione loss. Eur J Pharmacol 487:81–91
Arthur PG, Lim SCC, Meloni BP, Munns SE, Chan A, Knuckey NW (2004) The protective effect of hypoxic preconditioning on cortical neuronal cultures is associated with increases in the activity of several antioxidant enzymes. Brain Res 1017:146–154
Sen CK (1998) Redox signaling and the emerging therapeutic potential of thiol antioxidants. Biochem Pharmacol 55:1747–1758
Tsuji A, Tamai I (1999) Carrier-mediated or specialized transport of drugs across the blood–brain barrier. Adv Drug Deliv Rev 36:277–290
Neuwelt EA, Pagel MA, Hasler BP, Deloughery TG, Muldoon LL (2001) Therapeutic efficacy of aortic administration of N-acetylcysteine as a chemoprotectant against bone marrow toxicity after intracarotid administration of alkylators, with or without glutathione depletion in a rat model. Cancer Res 61:7868–7874
Kannan R, Chakrabarti R, Tang D, Kim KJ, Kaplowitz N (2000) GSH transport in human cerebrovascular endothelial cells and human astrocytes: evidence for luminal localization of Na+-dependent GSH transport in HCEC. Brain Res 852:374–382
Wang W, Ballatori N (1998) Endogenous glutathione conjugates: occurrence and biological functions. Pharmacol Rev 50:335–355
Campbell KC, Meech RP, Rybak LP, Hughes LF (2003) The effect of d-methionine on cochlear oxidative state with and without CDDP administration: mechanisms of otoprotection. J Am Acad Audiol 14:144–156
Campbell KC, Rybak LP, Meech RP, Hughes L (1996) d-methionine provides excellent protection from CDDP ototoxicity in the rat. Hear Res 102:90–98
Campbell KCM, Meech RP, Rybak LP, Hughes LF (1999) d-methionine protects against CDDP damage to the stria vascularis. Hear Res 138:13–28
Poirson-Bichat F, Bras Goncalves RA, Miccoli L, Dutrillaux B, Poupon MF (2000) Methionine depletion enhances the antitumoral efficacy of cytotoxic agents in drug-resistant human tumor xenografts. Clin Cancer Res 6:643–653
Wimmer C, Mees K, Stumpf P, Welsch U, Reichel O, Suckfull M (2004) Round window application of d-methionine, sodium thiosulfate, brain-derived neurotrophic factor, and fibroblast growth factor-2 in CDDP induced ototoxicity. Otol Neurotol 25:33–40
Sha SH, Schacht J (2000) Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: d-methionine is a potential protectant. Hear Res 142:34–40
Kopke RD, Coleman JKM, Liu J, Campbell KCM, Riffenburgh RH (2002) Enhancing intrinsic cochlear stress defenses to reduce noise-induced hearing loss. Laryngoscope 112:1515–1532
Dickey DT, Muldoon LL, Kraemer DF, Neuwelt EA (2004) Protection against cisplatin-induced ototoxicity by N-acetylcysteine in a rat model. Hear Res 193:25–30
Doolittle ND, Muldoon LL, Brummett RE, Tyson RM, Lacy C, Bubalo JS et al (2001) Delayed sodium thiosulfate as an otoprotectant against carboplatin-induced hearing loss in patients with malignant brain tumors. Clin Cancer Res 7:493–500
Muldoon LL, Pagel MA, Kroll RA, Brummett RE, Doolittle ND, Zuhowski EG et al (2000) Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin Cancer Res 6:309–315
Wu YJ, Muldoon LL, Neuwelt EA (2005) The chemoprotective agent N-acetylcysteine blocks cisplatin-induced apoptosis through caspase signaling pathway. J Pharmacol Exp Ther 312:424–431
Brock P, Pritchard J, Bellman S, Pinkerton CR (1988) Ototoxicity of high-dose cisplatinum in children. Med Pediatr Oncol 16:368–369
Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J (1991) Cisplatin ototoxicity in children: a practical grading system. Med Pediatr Oncol 19:295–300
Pritchard J, Brown J, Shafford E, Perilongo G, Brock P, Dicks-Mireaux C et al (2000) Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach—results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol 18:3819–3828
Perilongo G, Shafford E, Maibach R, Aronson D, Brugieres L, Brock P et al (2004) International society of paediatric oncology-SIOPEL 2. Risk-adapted treatment for childhood hepatoblastoma. Final report of the second study of the International Society of Paediatric Oncology-SIOPEL 2. Eur J Cancer 40:411–421
Peters U, Preisler-Adams S, Hebeisen A, Hahn M, Seifert E, Lanvers C et al (2000) Glutathione S-transferase genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Drugs 11:639–643
Peters U, Preisler-Adams S, Lanvers-Kaminsky C, Jurgens H, Lamprecht-Dinnesen A (2003) Sequence variations of mitochondrial DNA and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Res 23:1249–1255
Neuwelt EA, Gilmer-Knight K, Lacy C, Nicholson HS, Kraemer DF, Doolittle ND et al (2005) Toxicity profile of delayed high-dose sodium thiosulfate in children treated with carboplatin in conjunction with blood–brain-barrier disruption. Pediatr Blood Cancer 47(2):174–182. DOI 10.1002/pbc.20529
Fine HA, Mayer RJ (1993) Primary central nervous system lymphoma. Ann Intern Med 119:1093–1104
Blay JY, Conroy T, Chevreau C, Thyss A, Quesnel N, Eghbali H et al (1998) High-dose methotrexate for the treatment of primary cerebral lymphomas: analysis of survival and late neurologic toxicity in a retrospective series. J␣Clin Oncol 16: 864–871
Abrey LE, DeAngelis LM, Yahalom J (1998) Long-term survival in primary CNS lymphoma. J Clin Oncol 16:859–863
DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ (2002) Combination chemotherapy and radiotherapy for primary central system lymphoma: radiation therapy oncology group study 93-10. J Clin Oncol 20:4643–4648
Maiese K, Li F, Chong ZZ (2004) Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci 25:577–583
Acknowledgments
This meeting was supported by the National Institutes of Health grant (R13 CA 86959-05) through the National Cancer Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute of Deafness and Other Communication Disorders.
We thank Aimee Mackillop, Nan Hedrick, Lisa Bennett, Charity Saul and Aliana Kim for coordinating the meeting, and the following for their presentations and for participating in the CNS metastases and chemoprotection discussions: Nancee Albright, Robert Albright, Lily Angelov, Nicholas Avgeropoulos, Gene Barnett, Sue Bell, Lisa Bennett, Susan Blaney, Archie Bleyer, Penelope Brock, Patti Bruns, Joseph Bubalo, Kathleen Campbell, Jonathan Carlson, Patricia Caviness, Gregory Christoforidis, Megan Daman, Tom Dickey, Amy Donahue, Nancy Doolittle, Sandy Ference, David Fortin, Joseph Frank, Kathy Gilliland, Kristy Gilmer-Knight, Jane Gordon, Mary Kay Gumerlock, Paul Gumerlock, Walter Hall, Marianne Haluska, Nan Hedrick, Maria Hopple, Paula Jacobs, Tom Jacobs, Dale Kraemer, Outi Kuittinen, Cynthia Lacy, William Leenders, Manfred Lindau, Paul Lockman, A.B. Madhnakumar, Sadhan Majumder, Sandor Manninger, Diana Manninger-Solymosi, Lazlo Marik, Nathan McDannold, John McGregor, Margaret McMahon, Leslie Muldoon, Gary Nesbit, Edward Neuwelt, H. Stacy Nicholson, Alfred Nuttall, Brian O’Neill, Michael Pagel, Diane Palmieri, Joe Pancrazio, William Pardridge, David Peereboom, Darryl Peterson, Brad Pollock, Marie-Eve Potvin, Stephen Rayhill, Kelly Reavis, Rae Rosenberg, Axel Rosengart, Chloe Sandquist, Edna Shalom, Tali Siegal, Quentin Smith, Lisa Sorenson, Carole Soussain, Alexander Spence, Glen Stevens, Paul Tratnyek, John Trusheim, Rose Marie Tyson, Kishena Wadhwani, Shannan Walker-Rosenfeld, Roy Wu, Jeffrey Wu, Byron Young, Carol Saunders Young.
Author information
Authors and Affiliations
Corresponding author
Additional information
OHSU, Portland Veterans Affairs Medical Center (PVAMC) and the Department of Veterans Affairs have a significant financial interest in Adherex, a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest was reviewed and managed by the OHSU Integrity Program Oversight Council and the PVAMC Conflict of Interest in Research Committee.
Rights and permissions
About this article
Cite this article
Doolittle, N.D., Peereboom, D.M., Christoforidis, G.A. et al. Delivery of chemotherapy and antibodies across the blood–brain barrier and the role of chemoprotection, in primary and metastatic brain tumors: report of the eleventh annual blood–brain barrier consortium meeting. J Neurooncol 81, 81–91 (2007). https://doi.org/10.1007/s11060-006-9209-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-006-9209-y