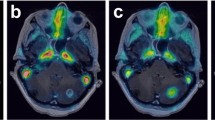
Summary
Purpose
Motexafin gadolinium (MGd) is an investigational pharmaceutical with radiation enhancing properties. Magnetic Resonance Imaging (MRI) was used to measure brain and tumor MGd levels to evaluate (1) the degree to which MGd passes through the intact blood brain barrier, and (2) the retention of MGd in tumor in patients with glioblastoma multiforme (GBM).
Methods and materials
MRI studies were performed on GBM patients who participated in a phase I clinical trial in which MGd was given during standard fractionated radiation therapy. MGd was administered daily (Monday to Friday) for five or 10 doses as a loading regimen, followed by three times per week dosing as a maintenance schedule. T1-weighted MRI was performed at intervals throughout the course of the MGd administration and radiation therapy in the 33 participating patients. Eleven patients had pre- and post-MGd scans, allowing for study of MGd's normal blood brain barrier penetration. Twenty-two patients had adequate residual tumor for measurements to evaluate MGd retention in tumor during the course of MGd and radiation administration.
Results and conclusions
The studies of uninvolved brain tissue support the conclusion that MGd does not cross the intact blood brain barrier in detectable quantities. The tumor study showed MGd uptake during loading and maintenance without measurably significant fall off on non-dosage days during the maintenance dosing. Although the number of cases is small, the 10-day loading regimen showed greater drug loading and retention compared with the 5 days loading regimen.
Similar content being viewed by others
References
Ohgaki H, Kleihues P 2005 Epidemiology and etiology of gliomasActa Neuropathol 109:93–108
Curran WJ, Scott CB, Horton J, et al. 1993 Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trialsJ Nat Cancer Inst 85: 704–710
Simpson JR, Horton J, Scott C, et al. 1993 Influence of location and extend of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trailsInt J Radiat Oncol Biol Phys 26(2): 239–244
Walker MD, Alexander E, Jr., Hunt WE, et al. 1978 Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trialJ Neurosurg 49:333–343
Walker MD, Strike TA, Sheline GE 1979 An analysis of dose–effect relationship in the radiotherapy of malignant gliomasInt J Radiat Oncol Biol Phys 5:1725–1731
Steward LA, 2002 Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomized trialsLancet 359: 1011–1018
Stupp R, Dietrich PY, Ostermann KS, et al. 2002: Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomideJ Clin Oncol 20(5): 1375–1382
Brada M, Hoang-Xuan K, Rampling R, et al. 2001 Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapseAnn Oncol 12(2): 259–266
Lanzetta G, Campanella C, Rozzi A, et al. 2003 Temozolomide in radio-chemotherapy combined treatment for newly-diagnosed glioblastoma multiforme: phase II clinical trialAnticancer Res. 23(6D):5159–5164
Daumas-Duport C, Scheithauer BW, Kelly PJ 1987 A histologic and cytologic method for the spatial definition of gliomasMayo Clin Proc 62: 435–449
Earnest F IV, Kelly PJ, Scheithauer BW, et al. 1988 Cerebral astrocytomas: histopathologic correlation of MR and CT contrast enhancement with stereotactic biopsy. Radiology 166(3): 823–827
Sessler JL, Murai T, Hemmi G 1989 A water-stable Gadolinium (III) complex derived from a new pentadentate “expanded porphyrin” ligandInorg Chem 28: 3390–3393
Sessler JL, Burrell AK 1992 Expanded porphyrins Topics Curr Chem 161: 177–273
Young SW, Qing F, Harriman A, et al. 1996 Gadolinium (III) texaphyrin: a tumor selective radiation sensitizer that is detectable by MRIProc Natl Acad Sci USA 93: 6610–6615
Sessler JL, Mody TD, Hemmi GW, et al. 1993 Gadolinium (III) texaphyrin: a novel MRI contrast agentJ Am Chem Soc 115: 10368–10369
Young SW, Sidhu MK, Qing F, et al. 1994 Preclinical evaluation of gadolinium (III) texaphyrin complexInv Radiol 29: 330–338
Sessler JL, Miller RA 2000 Texaphyrins: new drugs with diverse clinical applications in radiation and photodynamic therapyBiochem Pharmacol 59:733–739
Haacke EM, Brown RW, Thompson MR, Venkatesan R 1999 Magnetic Resonance Imaging – Physical Principles and Sequences Design. John Wiley & Sons, Inc., New York, p 367–370
Sessler JL,Tvermoes NA, Guldi DM, et al. 1999 One-electron reduction and oxidation studies of the radiation sensitizer gadolinium(III) texaphyrin (PCI-0120) and other water soluble metallotexaphyrinsJ Phys Chem A 103: 787–794
Magda D, Lepp C, Gerasimchuk N, et al. 2001 Redox cycling by motexafin gadolinium enhances cellular response to ionizing radiation by forming reactive oxygen speciesInt J Radiat Oncol Biol Phys 51: 1025–1036
Xu S, Zakian K, Thaler H, et al. 2001 Effect of motexafin gadolinium on tumor metabolism and radiation sensitivityInt J Radiat Oncol Biol Phys 49: 1381–1390
Miller RA, Woodburn K, Qing F, et al. 1999 In vivo animal studies with gadolinium (III) texaphyrin as a radiation enhancerInt J Radiat Oncol Biol Phys 45: 981–989
Mehta MP, Gius DR, Rockwell S, Thomas JP: Redox modulation: a novel approach to potentiate the effect of radiation therapy. A CME-certified symposium immediately preceding the 2001 annual meeting of ASTRO, San Francisco, November 3, 2001
Donnelly ET, Liu YF, Fatunmbi YO, et al. 2004 Effects of texaphyrins on the oxygenation of EMT6 mouse mammary tumorsInt J Radiat Oncol Biol Phys 58(5): 1570–1576
Mody TD, Fu L, Sessler JL 2001 Texaphyrins: synthesis and development of a novel class of therapeutic agents In: Karlin KD, eds Progress in Inorganic Chemistry. John Wiley & Sons Inc, New York, pp. 551–598
Sessler JL, Mody TD, Hemmi GW, et al. 1993 Synthesis and structural characterization of Lanthanide(III) TexaphyrinsInorg Chem 32: 3175–3187
Sessler JL, Hemmi G, Mody TD, et al. 1994 Texaphyrins: synthesis and applicationsAcc Chem Res 27: 43–50
Woodburn KW, 2001 Intracellular localization of the radiation enhancer Motexafin Gadolinium using interferometric Fourier fluorescence microscopyJ Pharmacol Exp Ther 297: 888–894
Viala J, Vanel D, Meingan P, et al. 1999 Phases IB and II multidose trial of Gadolinium Texaphyrin, a radiation sensitizer detectable at MR imaging: preliminary results in brain metastasesRadiology 212: 755–759
Mehta MP, Shapiro WR, Glantz MJ, et al. 2002 Lead-in phase to randomized trial of Motexafin Gadolinium and whole-brain radiation for patients with brain metastases: centralized assessment of magnetic resonance imaging, neurocognitive, and neurologic end pointsJ Clin Oncol 20: 3445–3453
Carde P, Timmerman R, Mehta MP, et al. 2001 Multicenter phase Ib/II trial of the radiation enhancer Motexafin gadolinium in patients with brain metastasesJ Clin Oncol 19: 2074–2083
Mehta MP, Rodrigus P, Terhaard CH, et al. 2003 Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastasesJ Clin Oncol 21(13): 2529–2536
Meyers CA, Smith JA, Bezjak A, et al. 2004 Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trialJ Clin Oncol 22(1):157–165
Rosenthal DI, Nurenberg P, Becerra CR, et al. 1999 A Phase I single-dose trial of Gadolinium Texaphyrin (Gd-Tex), a tumor selective radiation sensitizer detectable by magnetic resonance imagingClin Cancer Res 5: 739–745
Woods RP, Cherry SR, Mazziotta JC 1992 Rapid automated algorithm for aligning and reslicing PET imagesJ Comput Assist Tomogr 16:620–633
Newman HF, Bleehen NM, Ward R, et al. 1988 Hypoxic cell radiosensitizers in the treatment of high grade gliomas: a new direction using combined Ro 03–8799 (pimonidazole) and SR 2508 (etanidazole)Int J Radiat Oncol Biol Phys 15: 677–684
Henry RG, Vigneron DB, Eischbein NJ, et al. 2000 Comparison of relative cerebral blood volume and proton spectroscopy in patients with treated gliomasAm J Neuroradiol 21(2): 357–366
Manon R, Hui S, Chinnaiyan P, et al. 2004 The impact of mid-treatment MRI on defining boost volumes in the radiation treatment of glioblastoma multiformeTechnol Cancer Res Treat 3(3):303–307
Acknowledgements
This research has been supported by the National Cancer Institute (Cancer Therapy Evaluation Program, CA78170 and the supplement to UCLA Johnson Comprehensive Cancer Center Core Grant), the UCLA General Clinical Research Center and Pharmacyclics Inc. Image processing and analysis was performed in the Ahmanson-Lovelace Brain Mapping Center with the support of the following organizations and grants: Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Northstar Fund, and the National Center for Research Resources grants RR12169, RR13642 and RR08655.
Tom Lai, Andrew Frew, Anne Wagner and Jenaro Felix have provided technical assistance. Dale Miles from Pharmacyclics Inc. provided blood sample data for the participating patients.
Meeting presentations:
The blood brain barrier investigation was presented at the 45th American Society of Therapeutic Radiology and Oncology, October 2003, Salt Lake City, USA. The MGd tumor retention study was presented at the 9th annual meeting of Society of Neuro Oncology, November 2004, Toronto, Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, G.N., Ford, J.M. & Alger, J.R. MRI measurement of the uptake and retention of motexafin gadolinium in glioblastoma multiforme and uninvolved normal human brain. J Neurooncol 77, 95–103 (2006). https://doi.org/10.1007/s11060-005-9101-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-005-9101-1