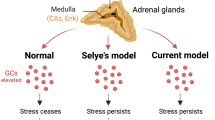
Data on the influences of stress on the function of long-term synaptic plasticity are analyzed. Using longterm potentiation (LTP) as an example, stress has been shown to have both stimulatory and inhibitory influences on the effectiveness of the induction of long-term modifications, the effect depending on the nature, duration, and intensity of the stress, the observation time point, the brain structure being studied, and, thus, the involvement in the stress response of the different mechanisms underlying LTP. Stress-induced increases in glucocorticoid levels did not obligately correlate with changes in long-term plasticity, while application of corticosterone in vivo and in vitro could lead to both activation and inhibition of LTP. Existing data provide evidence that changes in LTP are determined by the ratio of mineralocorticoid and glucocorticoid receptors, activation of the latter not so much impairing the mechanisms of generation as increasing the threshold of induction of LTP, regulating the metaplasticity of synapses. The unpredictability of the effects of stress is related in particular to the involvement of other transmitter systems regulating metaplasticity whose actions depend on the animal’s individual experience in the stress reaction. The range of individual differences stimulates the ongoing search for significant factors determining the stress reactivity of longterm plasticity underlying stress resistance or susceptibility to its pathological consequences. Differences in the processing of signals arriving at neurons and their molecular mediation may constitute such a factor.
Similar content being viewed by others
References
Ahmed, T., Frey, J. U., and Korz, V., “Long-term effects of brief acute stress on cellular signaling and hippocampal LTP,” J. Neurosci., 26, 3951–3958 (2006).
Akirav, I. and Richter-Levin, G., “Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat,” J. Neurosci., 19, 10,530–10,535 (1999).
Akirav, I. and Richter-Levin, G., “Mechanisms of amygdala modulation of hippocampal plasticity,” J. Neurosci., 22, 9912–9921 (2002).
Aldenhoff, J. B., Gruol, D. L., Rivier, J., et al., “Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons,” Science, 221, 875–877 (1983).
Aleisa, A. M., Alzoubi K. H., Gerges, N. Z., and Alkadhi, K. A., “Nicotine blocks stress-induced impairment of spatial memory and long-term potentiation of the hippocampal CA1 region,” Int. J. Neuropsychopharmacol., 9, No. 4, 417–426 (2006).
Alfarez, D. N., Wiegert, O., Joëls, M., and Krugers, H. T. “Corticosterone and stress reduce synaptic potentiation in mouse hippocampal slices with mild stimulation,” Neuroscience, 115, No. 4, 1119–1126 (2002).
Arima-Yoshida, E., Watabe, A. M., and Manabe, T., “The mechanisms of the strong inhibitory modulation of long-term potentiation in the rat dentate gyrus,” Eur. J. Neurosci., 33, 1637–1646 (2011).
Artola, A., von Frijtag, J. C., Fermont, P. C., et al., “Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment,” Eur. J. Neurosci., 23, No. 1, 261–272 (2006).
Avital, A., Segal, M., and Richter-Levin, G., “Contrasting roles of corticosteroid receptors in hippocampal plasticity,” J. Neurosci., 26, 9130–9134 (2006).
Basta-Kaim, A., Szczesny, E., Glombik, K., et al., “Prenatal stress leads to changes in IGF-1 binding proteins network in the hippocampus and frontal cortex of adult male rat,” Neuroscience, 274, 59–68 (2014).
Blank, T., Nijholt, L., and Spiess, J., “Molecular determinants mediating effects of acute stress on hippocampus-dependent synaptic plasticity and learning,” Mol. Neurobiol., 29, 131–138 (2004).
Blank, T., Nijholt, L., Eckart, K., and Spiess, J., “Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning,” J. Neurosci., 22, 3788–3794 (2002).
Bobula, B., Sowa, J., and Hess, G., “Anti-interleukin-1 antibody prevents the occurrence of repeated restraint stress-induced alterations in synaptic transmission and long-term potentiation in the rat frontal cortex,” Pharm Rep., 67, No. 1, 123–128 (2015).
Bobula, B., Wabno, J., and Hess, G., “Imipramine counteracts corticosterone-induced enhancement of glutamatergic transmission and impairment of long-term potentiation in the rat frontal cortex,” Pharm. Rep., 63, 1404–1412 (2011).
Boume, J. N. and Harris, K. M., “Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP,” Hippocampus, 21, 354–373 (2011).
Bramham, C. R., Southard, T., Ahlers S., T., and Sarvey, J. M., “Acute cold stress leading to elevated corticosterone neither enhances synaptic efficacy nor impairs LTP in the dentate gyrus of freely moving rats,” Brain Res., 789, No. 2, 245–255 (1998).
Brunson, K. L., Kramar, E., Lin, B., Chen, Y., et al., “Mechanisms of late-onset cognitive decline after early-life stress,” J. Neurosci., 25, No. 41, 9328–9338 (2005).
Cazakoff, B. N. and Howland, J. G., “Acute stress disrupts paired pulse facilitation and long-term potentiation in rat dorsal hippocampus through activation of glucocorticoid receptors,” Hippocampus, 20, 1327–1331 (2010).
Cestari, V., Rossi-Arnaud, C., Saraulli, D., and Costanzi, M., “The MAP(K) of fear: from memory consolidation to memory extinction,” Brain Res. Bull., 105, 8–16 (2014).
Chameau, P., Qin, Y., Spijker, S., et al., “Glucocorticoids specifically enhance L-type calcium current amplitude and affect calcium channel subunit expression in the mouse hippocampus,” J. Neurophysiol., 97, No. 1, 5–14 (2007).
Champagne, D. L., Bagot, R. C., van Hasselt, E., et al., “Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress,” J. Neurosci., 28, No. 23, 6037–6045 (2008).
Charil, A., Laplante, D. P., Vaillancourt, C., and King, S., “Prenatal stress and brain development,” Brain Res. Rev., 65, 56–79 (2010).
Chen, J. L., Lin, W. C., Cha J., W., So, P. T., et al.,“ Structural basis for the role of inhibition in facilitating adult brain plasticity,” Nat. Neurosci., 14, 587–594 (2011).
Chen, Y., Fenoglio, K. A., Dubé, C. M., et al., “Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent,” Mol. Psychiatry, 11, No. 11, 992–1002 (2006).
Chiuccariello, L., Houle, S., Miler, L., et al., “Elevated monoamine oxidase a binding during major depressive episodes is associated with greater severity and reversed neurovegetative symptoms,” Neuropsycho pharmacology, 39, No. 4, 973–980 (2014).
Conboy, L. and Sandi, C., “Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action,” Neuropsychopharmacology, 35, 674–685 (2010).
de Kloet, E. R., Joels, M., and Holsboer, E., “Stress and the brain: from adaptation to disease,” Nat. Rev. Neurosci., 6, No. 6, 463–475 (2005).
de Kloet, E. R., Oitzl, M. S., and Jols, M., “Stress and cognition: are corticosteroids good or bad guys?” Trends Neurosci., 22, No. 10, 422–426 (1999).
Diamond, D. M., Bennett, M. C., Fleshner, M., and Rose, G. M., “Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation,” Hippocampus, 2, No. 4, 421–430 (1992).
Diamond, D. M., Park, C. R., and Woodson, J. C., “Stress generates emotional memories and retrograde amnesia by inducing an endogenous form of hippocampal LTP,” Hippocampus, 14, No. 3, 281–291 (2004).
Diamond, D. M., Park, C. R., Campbell, A. M., and Woodson, J. C., “Competitive interactions between endogenous LTD and LTP in the hippocampus underlie the storage of emotional memories and stress-induced amnesia,” Hippocampus, 15, No. 8, 1006–1025 (2005).
Dreyer, B. D., Riedel, G., and Platt, B., “The cholinergic system and hippocampal plasticity,” Behav. Brain Res., 221, 505–514 (2011).
Fa, M., Xia, L., Anunu, R., et al., “Stress modulation of hippocampal activity-spotlight on the dentate gyrus,” Neurobiol. Learn. Mem, 112, 53–60 (2014).
Fabricius, K., Wörtwein, G., and Pakkenberg, B., “The impact of maternal separation on adult mouse behaviour and on the total neuron number in the mouse hippocampus,” Brain Struct. Funct., 212, 403–416 (2008).
Feldman, D. E., “The spike-timing dependence of plasticity,” Neuron, 75, 556–571 (2012).
Finsterwald, C. and Alberini, C. M., “Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies,” Neurobiol. Learn. Mem, 112, 17–29 (2014).
Fontella, F. U., Vendite, D. A., Tabajara, A. S., et al., “Repeated restraint stress alters hippocampal glutamate uptake and release in the rat,” Neurochem. Res., 29, No. 9, 1703–1709 (2004).
Franklin, T. B., Linder, N., Russig, H., et al., “Influence of early stress on social abilities and serotonergic functions across generations in mice,” PLoS One, 6, e21842 (2011).
Frey, S., Bergado-Rosado, J., Seindenbecher, T., et al., “Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: heterosynaptic induction mechanisms of late-LTP,” J. Neurosci., 21, 3697–3703 (2001).
Fritschy, J. and Panzanelli, P., “GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system,” Eur. J. Neurosci., 39, 1845–1865 (2014).
Gadek-Michalska, A. and Bugajski, J., “Repeated handling, restraint, or chronic crowding impair the hypothalamic pituitary-adrenocortical response to acute restraint stress,” J. Physiol. Pharmacol., 54, 449–459 (2003).
Gibbs, M. E., Hutchinson, D. S., and Summers, R. J., “Role of betaadrenoceptors in memory consolidation: beta3-adrenoceptors act on glucose uptake and beta2-adrenoceptors on glycogenolysis,” Neuropsycho pharmacology, 33, 2384–2397 (2008).
Godukhin, O. V., “The role of cytokines in the development of convulsive activity in the brain,” Zh. Vyssh. Nerv. Deyat., No. 5, 541–552 (2007).
Grigor’yan, G. A. and Gulyaeva, N. V., “Stress reactivity and stress resistance in the pathogenesis of depressive disorders: the role of epigenetic mechanisms,” Zh. Vyssh. Nerv. Deyat., 65, No. 1, 19–32 (2015).
Grigor’yan, G. A., Dygalo, N. N., Gekht, A. B., et al., “Molecular cellular mechanisms of depression. The roles of glucocorticoids, cytokines, neurotransmitters, and trophic factors in the genesis of depressive disorders,” Usp. Fiziol. Nauk, 45, No. 2, 3–19 (2014).
Grigoryan, G., Ardi, Z., Albrecht, A., et al., “Juvenile stress alters LTP in ventral hippocampal slices: involvement of noradrenergic mechanisms,” Behav. Brain Res., 278, 559–562 (2015).
Groc, L., Choquet, D., and Chaouloff, E., “The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation,” Nat. Neurosci., 11, 868–870 (2008).
Groeneweg, E. L., Karst, H., de Kloet, E. R., and Joels, M., “Rapid nongenomic effects of corticosteroids and their role in the central stress response,” J. Endocrinol., 209, 153–167 (2011).
Grunewald, M., Johnson, S., Lu, D., et al., “Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression,” J. Biol. Chem., 287, No. 29, 24195–24206 (2012).
Gulyaeva, N. V., “Fundamental and translational aspects of the stress reactivity of the ventral hippocampus: functional biochemical mechanisms of changes in neuroplasticity,” Neirokhimiya, No. 2, 101–111 (2015).
Hayama, T., Noguchi, J., Watanabe, S., et al., “GABA promotes the competitive selection of dendritic spins by controlling local Ca2+ signaling,” Nat. Neurosci., 16, No. 10, 1409–1416 (2013).
Hewitt, S. A., Wamsteeker, J. I., Kurz E.,U., and Bains, J. S., “Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis,” Nat. Neurosci., 12, 438–443 (2009).
Hiraide, S., Saito, Y., Matsumoto, M., et al., “Possible modulation, of the amygdala on metaplasticity deficits in the hippocampal CA1 field in early postnatally stressed rats,” J. Pharmacol. Sci., 119, 64–72 (2012).
Hirata, R., Matsumoto, M., Judo, C., et al., “Possible relationship between the stress-induced synaptic response and metaplasticity in the hippocampal CA1 field of freely moving rats,” Synapse, 63, No. 7, 549–556 (2009).
Holm, M. M., Nieto-Gonzalez, I. L., Vardya, I., et al., “Hippocampal GABA - ergic dysfunction in a rat chronic mild stress model of depression,” Hippocampus, 21, No. 4, 422–433 (2011).
Holmes, A. and Wellman, C. L., “Stress-induced prefrontal reorganization and executive dysfunction in rodents,” Neurosci. Biobehav. Rev., 33, 773–783 (2009).
Howland, I. G. and Wang, Y. T., “Synaptic plasticity in learning and memory: stress effects in the hippocampus,” Prog. Brain Res., 169, 145–158 (2008).
Hu, W., Zhang, M., Czeh, B., et al., “Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network,” Neuropsychopharmacology, 35, 1693–1707 (2010).
Huang, C. C., Yang, C. H., and Hsu, K. S., “Do stress and long-term potentiation share the same molecular mechanisms?” Mol. Neurobiol., 32, No. 3, 223–235 (2005).
Inoue, W. and Bains, J. S., “Beyond inhibition: GABA synapses tune the neuroendocrine stress axis,” Bioessays, 36, 561–569 (2014).
Inoue, W., Baimoukhametova D. V., Fuzesi, T., et al., “Noradrenaline is a stress-associated metaplastic signal at GABA synapses,” Nat. Neurosci., 16, No. 5, 605–612 (2013).
Ishikawa, S., Saito, Y., Yanagawa, Y., et al., “Early postnatal stress alters extracellular signal-regulated kinase signaling in the corticolimbic system modulating emotional circuitry in adult rats,” Eur. J. Neurosci., 35, No. 1, 135–145 (2012).
Jin, Y., Kanno, T., and Nishizaki, T., “Acute restraint stress impairs induction of long-term potentiation by activating GSK3β,” Neurochem. Res., 40, No. 1, 36–40 (2015).
Joels, M. and Krugers, H. T., “LTP after Stress: Up or Down?” Neural Plast., 2007, 93202 (2007).
Joels, M., “Corticosteroid effects in the brain: U-shape it,” Trends Pharmacol. Sci., 27, 5, 244–250 (2006).
Joels, M., “Stress, the hippocampus, and epilepsy,” Epilepsia, 50, 586–597 (2009).
Joels, M., Karst, H., DeRijk, R., and de Kloet, E. R., “The coming out of the brain mineralocorticoid receptor,” Trends Neurosci., 31, No. 1, 1–7 (2008).
Joels, M., Krugers, H. J., Lucassen, P. I., and Karst, H., “Corticosteroid effects on cellular physiology of limbic cells,” Brain Res., 293, 91–100 (2009).
Joels, M., Velzing, E., Nair, W., et al., “Acute stress increases calcium current amplitude in rat hippocampus: temporal changes in physiology and gene expression,” Eur. J. Neurosci., 18, 1315–1324 (2003).
Kallarackal, A. J., Kvarta, M. D., Cammarata, E., et al., “Chronic stress induces a selective decrease in AMPA receptor-mediated synaptic excitation at hippocampal temporoammonic-CA1 synapses,” J. Neurosci., 33, No. 40, 15,669–15,674 (2013).
Kamal, A., Ramakers, G. M., Altinbilek, B., and Kas, M. J., “Social isolation stress reduces hippocampal long-term potentiation: effect of animal strain and involvement of glucocorticoid receptors,” Neuroscience, 256, 262–270 (2014).
Karst, H., Berger, S., Erdmann, G., et al., “Metaplasticity of amygdalar responses to the stress hormone corticosterone,” Proc. Natl. Acad. Sci. USA, 107, 14449–14454 (2010).
Karst, H., Berger, S., Turiault, M., et al., “Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone,” Proc. Natl. Acad. Sci. USA, 102, No. 52, 19204–19207 (2005).
Karst, H., Wadman, W. J., and Joëls, M., “Corticosteroid receptor-dependent modulation of calcium currents in rat hippocampal CA1 neurons,” Brain Res., 649, No. 1–2, 234–242 (1994).
Kavushansky, A., Vouimba, R. M., Cohen, H., and Richter-Levin, G., “Acti vity and plasticity in the CA1, the dentate gyrus, and the amygdala following controllable vs. uncontrollable water stress,” Hippocampus, 16, No. 1, 35–42 (2006).
Kim Ji, Song E.,Y. , and Kosten, T. A.,“ Stress effects in the hippocampus: synaptic plasticity and memory,” Stress, 9, 1–11 (2006).
Kim, J. J., Koo, J. W., Lee, H. J., and Han, J. S., “Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory,” J. Neurosci., 25, No. 6, 1532–1539 (2005) .
Kleschevnikov, A. M., Belichenko, P. V., Villar, A. J., et al., “Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome,” J. Neurosci., 24, 8153–8160 (2004).
Kleshchevnikov, A. M. and Voronin, L. L., “Repeated induction of longterm potentiation after its saturation in living hippocampal slices from rats,” Dokl. Akad. Nauk, 340, No. 5, 694–696 (1995).
Korz, V. and Frey, J. U., “Stress-related modulation of hippocampal longterm potentiation in rats: involvement of adrenal steroid receptors,” J. Neurosci., 23, No. 19, 7281–7287 (2003).
Krugers H. J., Alfarez, D. N., Karst, H., et al., “Corticosterone shifts different forms of synaptic potentiation in opposite directions,” Hippocampus, 15, No. 6, 697–703 (2005).
Kudryashova, I. V., “Plasticity of inhibitory synapses as a factor in longterm modifications,” Neirokhimiya, 32, No. 3, 181–191 (2015).
Lanfumey, L., Mongeau, R., Cohen-Salmon, C., and Hamon, M., “Corticos teroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders,” Neurosci. Biobehav. Rev., 32, 1174–1184 (2008).
Lapiz, M. D., Fulford, A., Muchimapura, S., et al., “Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission ,” Neurosci. Behav. Physiol., 33, No. 1, 13–29 (2003).
Lee, E. J., Son, G. H., Chung, S., et al., “Impairment of fear memory consolidation in maternally stressed male mouse offspring: Evidence for nongenomic glucocorticoid action on the amygdala,” J. Neurosci., 31, No. 19, 7131–7140 (2011).
Lee, S. Y., Hwang, Y. K., Yun, H. S., and Han, J. S., “Decreased levels of nuclear glucocorticoid receptor protein in the hippocampus of aged Long-Evans rats with cognitive impairment,” Brain Res., 1478, 48–54 (2012).
Lim, C. S., Kim Yi, Hwang, Y. K., et al., “Decreased interactions in protein kinase A-Glucocorticoid receptor signaling in the hippocampus after selective removal of the basal forebrain cholinergic input,” Hippocampus, 22, 455–465 (2012).
Liu, M., Li, J., Dai, P., et al., “Microglia activation regulates GluR1 phosphorylation in chronic unpredictable stress-induced cognitive dysfunction,” Stress, 18, No. 1, 96–106 (2015).
Lopes Aguiar, C., Romcy-Pereira, R. N., Escorsim Szawka, R., et al., “Mus carinic acetylcholine neurotransmission enhances the latephase of long-term potentiation in the hippocampal-prefrontal cortex pathway of rats in vivo: a possible involvement of monoaminergic systems,” Neuroscience, 153, No. 4, 1309–1319 (2008).
Lupien, S. J., McEwen, B. S., Gunnar, M. R., and Heim, C., “Effects of stress throughout the lifespan on the brain, behavior and cognition,” Nat. Rev. Neurosci., 10, 434–445 (2009).
Lussier, A. L., Romay-Tallon, R., Caruncho, H. J., and Kalynchuk, L. E., “Altered GABAergic and glutamatergic activity within the rat hippocampus and amygdala in rats subjected to repeated corticosterone administration but not restraint stress,” Neuroscience, 231, 38–48 (2013).
Maccari, S., Krugers, H. J., Morley-Fletcher, S., et al., “The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations,” J. Neuroendocrinol., 26, 707–723 (2014).
MacDougall, M. J. and Howland, J.G., “Acute stress and hippocampal output: exploring dorsal CA1 and subicular synaptic plasticity simultaneously in anesthetized rats,” Physiol. Rep., 1, No. 2, e00035 (2013).
Maggio, N. and Segal, M., “Cellular basis of a rapid effect of mineralocorticosteroid receptors activation on LTP in ventral hippocampal slices,” Hippocampus, 22, No. 2, 267–275 (2012).
Maggio, N. and Segal, M., “Differential corticosteroid modulation of inhibitory synaptic currents in the dorsal and ventral hippocampus,” J. Neurosci., 29, No. 9, 2857–2866 (2009a).
Maggio, N. and Segal, M., “Differential modulation of long-term depression by acute stress in the rat dorsal and ventral hippocampus,” J. Neurosci., 29, No. 27, 8633–8638 (2009b).
Maggio, N. and Segal, M., “Striking variations in corticosteroid modulation of long-term potentiation along the septotemporal axis of the hippocampus,” J. Neurosci., 27, No. 21, 5757–5765 (2007).
Maguire, J. and Mody, I., “Steroid hormone fluctuations and GABA(A)R plasticity,” Psychoneuroendocrinology, 34, S84–S90 (2009).
Marmigere, E., Givalois, L., Rage, E., et al., “Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats,” Hippocampus, 13, 646–655 (2003).
Maroun, M., “Stress reverses plasticity in the pathway projecting from the ventromedial prefrontal cortex to the basolateral amygdala,” Eur. J. Neurosci., 24, No. 10, 2917–2922 (2006).
Martin, S., Henley, J. M., Holman, D., et al., “Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity,” PLoS One, 4, No. 3, e4714 (2009).
Matsumoto, K., Puia, G., Dong, E., and Pinna, G., “GABA(A) receptor neu ro transmission dysfunction in a mouse model of social isolation-induced stress: possible insights into a non-serotonergic mechanism of action of SSRIs in mood and anxiety disorders,” Stress, 10, 3–12 (2007).
Matsumoto, M., Togashi, H., Ohashi, S., et al., “Serotonergic modulation of psychological stress-induced alteration in synaptic plasticity in the rat hippocampal CA1 field,” Brain Res., 1022, 221–225 (2004).
McEwen, B. S., “Physiology and neurobiology of stress and adaptation: central role of the brain,” Physiol. Rev., 87, 873–904 (2007).
McGaugh, J. L., “Making lasting memories: remembering the significant,” Proc. Natl. Acad. Sci. USA, 110, No. 2, 10402–10407 (2013).
McReynolds, J. R., Donowho, K., Abdi, A., et al., “Memory-enhancing corticosterone treatment increases amygdala norepinephrine and Arc protein expression in hippocampal synaptic fractions,” Neurobiol. Learn. Mem, 93, 312–321 (2010).
Mesquita, A. R., Pêgo, J. M., Summavielle, T., et al., “Neurodevelopment milestone abnormalities in rats exposed to stress in early life,” Neuroscience, 147, 1022–1033 (2007).
Mora, E., Segovia, G., Del Arco, A., et al., “Stress, neurotransmitters, corticosterone and body-brain integration,” Brain Res., 1476, 71–85 (2012).
Musazzi, L., Racagni, G., and Popoli, M., “Stress, glucocorticoids and glutamate release: Effects of antidepressant drugs,” Neurochem. Int., 59, 138–149 (2011).
Nikzad, S., Vafaei, A. A., Rashidy-Pour, A., and Haghighi, S. “Systemic and intrahippocampal administrations of the glucocorticoid receptor antagonist RU38486 impairs fear memory reconsolidation in rats,” Stress, 14, 459–464 (2011).
Olijslagers, J. E, de Kloet, E. R., Elgersma, Y., et al., “Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors,” Eur. J. Neurosci., 27, No. 10, 2542–2550 (2008).
Oomen, C. A., Soeters, H., Audureau, N., et al., “Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood,” J. Neurosci., 30, 6635–6645 (2010).
Paille, V., Fino, E., Du, K., et al., “GABAergic circuits control spike-timing-dependent plasticity,” J. Neurosci., 33, No. 22, 9353–9363 (2013).
Park, H. J., Lee, S., Jung, J. W, et al., “Glucocorticoid- and long-term stress-induced aberrant synaptic plasticity are mediated by activation of the glucocorticoid receptor ,” Arch Pharm. Res., 38, No. 6, 1204–1212 (2015).
Pavlides, C., Nivon, L. G., and McEwen, B. S., “Effects of chronic stress on hippocampal long-term potentiation,” Hippocampus, 12, 2, 245–257 (2002).
Polman, J. A., de Kloet, E. R., and Datson, N. A., “Two populations of glucocorticoid receptor-binding sites in the male rat hippocampal genome,” Endocrinology, 154, 1832–1844 (2013).
Popoli, M., Yan, Z., McEwen, B. S., and Sanacora, G., “The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission,” Nat. Rev. Neurosci., 13, 22–37 (2011).
Prager, E. M., Brielmaier, J., Bergstrom, H. C., et al., “Localization of mineralocorticoid receptors at mammalian synapses,” PLoS One, 5, No. 12, e14344 (2010).
Qin, X., Liu, Y., Zhu, M., and Yang, Z., “The possible relationship between expressions of TRP C3/5 channels and cognitive changes in rat model of chronic unpredictable stress,” Behav. Brain Res., 290, 180–186 (2015).
Radahmadi, M., Hosseini, N., and Nasimi, A., “Effect of chronic stress on short and long-term plasticity in dentate gyrus; study of recovery and adaptation,” Neuroscience, 280, 121–129 (2014).
Radley, J. J., Rocher, A. B., Janssen, W. G., et al., “Reversibility of apical dendritic retraction in the rat media] prefrontal cortex following repeated stress,” Exp. Neurol., 196, 199–203 (2005).
Rey, M., Carlier, E., Talmi, M., and Soumireu-Mourat, B., “Corticosterone effects on long-term potentiation in mouse hippocampal slices,” Neuroendocrinology, 60, 36–41 (1994).
Richter-Levin, G. and Maroun, M., “Stress and amygdala suppression of metaplasticity in the medial prefrontal cortex,” Cereb. Cortex, 20, No. 10, 2433–2441 (2010).
Richter-Levin, G., “The amygdala, the hippocampus, and emotional modulation of memory,” Neuroscientist, 10, No. 1, 31–39 (2004).
Roozendaal, B., “Systems mediating acute glucocorticoid effects on memory consolidation and retrieval,” Prog. Neuropsychopharmacol. Biol. Psychiatry, 27, No. 8, 1213–1223 (2003).
Roozendaal, B., McEwen, B. S., and Chattarji, S., “Stress, memory and the amygdala,” Nat. Rev. Neurosci., 10, 423–433 (2009).
Sachs, B. D., Ni, J. R., and Caron, M. G., “Sex differences in response to chronic mild stress and congenital serotonin deficiency,” Psychoneuro endocrinology, 40, 123–129 (2014).
Sarkar, J., Wakefield, S., Mackenzie, G., et al., “Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors,” J. Neurosci., 31, 18,198–18,210 (2011).
Schayek, R. and Maroun, M., “Differences in stress-induced changes in extinction and prefrontal plasticity in postweanling and adult animals,” Biol. Psychiatry, 78, No. 3, 159–166 (2015).
Schmidt, M. V., Abraham, W. C., Maroun, M., et al., “Stress-induced metaplasticity: from synapses to behavior,” Neuroscience, 250, 112–120 (2013).
Segal, M., Richter-Levin, G., and Maggio, N., “Stress-induced dynamic routing of hippocampal connectivity: a hypothesis,” Hippocampus, 20, 1332–1338 (2010).
Serra, M., Pisu M. G., Mostallino M. C., et al., “Changes in neuroactive steroid content during social isolation stress modulate GABAA receptor plasticity and function,” Brain Res. Rev., 57, 520–530 (2008).
Sharvit, A., Segal, M., Kehat, O., et al., “Differential modulation of synaptic plasticity and local circuit activity in the dentate gyrus and CA1 regions of the rat hippocampus by corticosterone,” Stress, 18, No. 3, 319–327 (2015).
Shen, H., Sabaliauskas, N., Sherpa, A., et al., “A critical role for alpha-4betadelta GABAA receptors in shaping learning deficits at puberty in mice ,” Science, 327, No. 5972, 1515–1518 (2010).
Skrebitskii V. G. and Shtark, M. B. , “Fundamental basics of nervous system plasticity,” Vestn. Ross. Akad. Med. Nauk., No. 9, 39–44 (2012).
Slotkin, T. A., Kreider, M. L., Tate, C. A., and Seidler, E. J., “Critical prenatal and postnatal periods for persistent effects of dexamethasone on serotonergic and dopaminergic systems,” Neuropsycho pharmacology, 31, 904–911 (2006).
Sousa, N., Cerqueira, J. J., and Almeida, O. F., “Corticosteroid receptors and neuroplasticity,” Brain Res. Rev., 57, No. 2, 561–570 (2008).
Sowa, J., Bobula, B., Glombik, K., et al., “Prenatal stress enhances excitatory synaptic transmission and impairs long-term potentiation in the frontal cortex of adult offspring rats,” PLoS One, 10, No. 3, e0119407 (2015).
Spyrka, J. and Hess, G., “Repeated restraint-induced modulation of longterm potentiation in the dentate gyrus of the mouse,” Brain Res., 1320, 28–33 (2010).
Spyrka, J., Danielewicz, J., and Hess, G., “Brief neck restraint stress enhances long-term potentiation and suppresses long-term depression in the dentate gyrus of the mouse,” Brain Res. Bull., 85, 363–367 (2011).
Tsoory, M. M., Vouimba, R. M., Akirav, I., et al., “Amygdala modulation of memory-related processes in the hippocampus: potential relevance to PTSD,” Prog. Brain Res., 167, 35–51 (2008).
Venzala, E., Garcia-Garcia, A. L., Elizalde, N., and Tordera, R. M., “Social vs. environmental stress models of depression from a behavioural and neurochemical approach,” Eur. Neuropsychopharmacol., 23, No. 7, 697–708 (2013).
Verkuyl, J. M., Karst, H., and Joas, M., “GABAergic transmission in the rat paraventricular nucleus of the hypothalamus is suppressed by corticosterone and stress,” Eur. J. Neurosci., 21, 113–121 (2005).
Viviani, B., Boraso, M., Valero, M., et al., “Early maternal deprivation immunologically primes hippocampal synapses by redistributing interleukin-1 receptor type I in a sex dependent manner,” Brain Behav. Immun., 35, 135–143 (2014).
Voronin, L., Byzov, A., Kleschevnikov, A., et al., “Neurophysiological analysis of long-term potentiation in mammalian brain,” Behav. Brain Res., 66, No. 1–2, 45–52 (1995).
Vouimba, R. M. and Richter-Levin, G., “Physiological dissociation in hippocampal subregions in response to amygdala stimulation,” Cereb. Cortex, 15, 1815–1821 (2005).
Vouimba, R. M., Yaniv, D., and Richter-Levin, G., “Glucocorticoid receptors and beta-adrenoceptors in basolateral amygdala modulate synaptic plasticity in hippocampal dentate gyrus, but not in area CA1,” Neuropharmacology, 52, 244–252 (2007).
Vouimba, R. M., Yaniv, D., Diamond, D., and Richter-Levin, G., “Effects of inescapable stress on LTP in the amygdala versus the dentate gyrus of freely behaving rats,” Eur. J. Neurosci., 19, 1887–1894 (2004).
Wang, X. D., Su, Y. A., Wagner, K. V., et al., “Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss,” Nat. Neurosci., 16, No. 6, 706–713 (2013).
Wiegert, O., Joëls, M., and Krugers, H. J., “Timing is essential for rapid effects of corticosterone on synaptic potentiation in the mouse hippocampus,” Learn. Mem., 13, No. 2, 110–113 (2006).
Wiegert, O., Pu, Z., Shor, S., et al., “Glucocorticoid receptor activation selectively hampers N-methyl-D-aspartate receptor dependent hippocampal synaptic plasticity in vitro,” Neuroscience, 135, 403–411 (2005).
Wong, D. L., Tai, T. C., Wong-Faull, D. C., et al., “Epinephrine: a shortand long-term regulator of stress and development of illness: a potential new role for epinephrine in stress,” Cell. Mol. Neurobiol., 32, No. 5, 737–748 (2012).
Woodin, M. A., Ganguly, K., and Poo, M., “Coincident pre-and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl-transporter activity,” Neuron, 39, 807–820 (2003).
Yamada, K., McEwen, B. S., and Pavlides, C., “Site and time dependent effects of acute stress on hippocampal long-term potentiation in freely behaving rats,” Exp. Brain Res., 152, No. 1, 52–59 (2003).
Yang, C.-H., Huang, C.-Ch., and Hsu, K.-S., “Behavioral stress modifies hippocampal synaptic plasticity through corticosterone-induced sustained extracellular signal-regulated kinase/mitogen-activated protein kinase activation,” J. Neurosci., 24, No. 49, 11029–11034 (2004).
Yang, E. C. and Liang, K. C., “Interactions of the dorsal hippocampus, medial prefrontal cortex and nucleus accumbens in formation of fear memory: difference in inhibitory avoidance learning and contextual fear conditioning,” Neurobiol. Learn. Mem., 112, 186–194 (2014).
Yang, P.-C., Yang, C.-H., Huang, C.-C., and Hsu, K.-S., “Phosphatidylinositol 3-kinase activation is required for stress protocol-induced modification of hippocampal synaptic plasticity,” J. Biol. Chem., 283, 2631–2643 (2008).
Yuen, E. Y., Liu, W., Karatsoreos, L. N., et al., “Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory,” Mol. Psychiatry, 16, No. 2, 156–170 (2011).
Zheng, C. and Zhang, T., “Synaptic plasticity-related neural oscillations on hippocampus-prefrontal cortex pathway in depression,” Neuro science, 292, 170–180 (2015).
Zheng, G., Zhang, X., Chen, Y., et al., “Evidence for a role of GABAA receptor in the acute restraint stress-induced enhancement of spatial memory,” Brain Res., 1181, 61–73 (2007).
Zhu, M. Y., Wang, W. P., Huang, J., et al., “Repeated immobilization stress alters rat hippocampal and prefrontal cortical morphology in parallel with endogenous agmatine and arginine decarboxylase levels,” Neurochem. Int., 53, 346–354 (2008).
Zitman, F. M., Lucas, M., Trinks, S., et al., “Dentate gyrus local circuit is implicated in learning under stress-a role for neurofascin,” Mol. Neurobiol., 53, No. 2, 842–850 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Vysshei Nervnoi Deyatel’nosti imeni I. P. Pavlova, Vol. 66, No. 4, pp. 414–428, July–August, 2016.
Rights and permissions
About this article
Cite this article
Kudryashova, I.V., Gulyaeva, N.V. “Unpredictable Stress”: Ambiguity of Stress Reactivity in Studies of Long-Term Plasticity. Neurosci Behav Physi 47, 948–959 (2017). https://doi.org/10.1007/s11055-017-0496-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-017-0496-x