Abstract
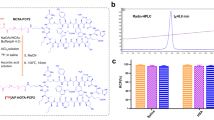
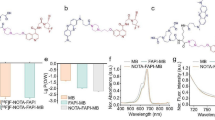
Ultrasmall superparamagnetic iron oxide nanoparticles (USPIOs) modified with a novel cyclic arginine-glycine-aspartate (RGD) peptide were made and radiolabeled as single-photon emission computed tomography (SPECT) and magnetic resonance imaging (MRI) dual-modality agents for imaging of breast cancer. The probe was tested both in vitro and in vivo to determine its receptor targeting efficacy and feasibility for SPECT and MRI. The radiochemical syntheses of 125I-cRGD-USPIO were accomplished with a radiochemical purity of 96.05 ± 0.33 %. High radiochemical stability was found in fresh human serum and in phosphate-buffered saline. The average hydrodynamic size of 125I-cRGD-USPIO determined by dynamic light scattering was 51.3 nm. Results of in vitro experiments verified the specificity of the radiolabeled nanoparticles to tumor cells. Preliminary biodistribution studies of 125I-radiolabeled cRGD-USPIO in Bcap37-bearing nude mice showed that it had long circulation half-life, high tumor uptake, and high initial blood retention with moderate liver uptake. In vivo tumor targeting and uptake of the radiolabeled nanoparticles in mice model were visualized by SPECT and MRI collected at different time points. Our results strongly indicated that the 125I-cRGD-USPIO could be used as a promising bifunctional radiotracer for early clinical tumor detection with high sensitivity and high spatial resolution by SPECT and MRI.











Similar content being viewed by others
References
Bouziotis P, Psimadas D, Tsotakos T, Stamopoulos D, Tsoukalas C (2012) Radiolabeled iron oxide nanoparticles as dual-modality SPECT/MRI and PET/MRI agents. Curr Top Med Chem 12:2694–2702
Brigger I, Dubernet C, Couvreur P (2002) Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev 54:631–651
Corot C, Robert P, Idée JM, Port M (2006) Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev 58:1471–1504
Curado MP (2011) Breast cancer in the world: incidence and mortality. Salud Publica Mex 53:372–384
Drukteinis JS, Mooney BP, Flowers CI, Gatenby RA (2013) Beyond mammography: new frontiers in breast cancer screening. Am J Med 126:472–479
Fang C, Veiseh O, Kievit F, Bhattarai N, Wang F, Stephen Z, Li C, Lee D, Ellenbogen RG, Zhang M (2010) Functionalization of iron oxide magnetic nanoparticles with targeting ligands: their physicochemical properties and in vivo behavior. Nanomedicine 9:1357–1369
Fani M, Psimadas D, Zikos C, Xanthopoulos S, Loudos GK, Bouziotis P, Varvarigou AD (2006) Comparative evaluation of linear and cyclic 99mTc-RGD peptides for targeting of integrins in tumor angiogenesis. Anticancer Res 26:431–434
Haubner R, Gratias R, Diefenbach B, Goodman SL, Jonczyk A, Kessler H (1996) Structural and functional aspects of RGD-containing cyclic pentapeptides as highly potent and selective integrin αvβ3 antagonists. J Am Chem Soc 118:7461–7472
Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL, Senekowitsch-Schmidtke R, Kessler H, Schwaiger M (2001) Noninvasive imaging of alpha(v)beta3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res 61:1781–1785
Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108:2064–2110
Lee HY, Li Z, Chen K, Hsu AR, Xu C, Xie J, Sun S, Chen X (2008) PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J Nucl Med 49:1371–1379
Li ZB, Wu Z, Chen K, Chin FT, Chen X (2007) Click chemistry for (18)F-labeling of RGD peptides and microPET imaging of tumor integrin alphavbeta3 expression. Bioconjug Chem 18:1987–1994
Lin RY, Dayananda K, Chen TJ, Chen CY, Liu GC, Lin KL, Wang YM (2012) Targeted RGD nanoparticles for highly sensitive in vivo integrin receptor imaging. Contrast Media Mol Imaging 7:7–18
Meng Q, Li Z (2013) Molecular imaging probes for diagnosis and therapy evaluation of breast cancer. Int J Biomed Imaging 2013:230487. doi:10.1155/2013/230487
Raynal I, Prigent P, Peyramaure S, Najid A, Rebuzzi C, Corot C (2004) Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest Radiol 39:56–63
Shi J, Kim YS, Zhai S, Liu Z, Chen X, Liu S (2009) Improving tumor uptake and pharmacokinetics of (64)Cu-labeled cyclic RGD peptide dimers with Gly(3) and PEG(4) linkers. Bioconjug Chem 20:750–759
Shokeen M, Fettig NM, Rossin R (2008) Synthesis, in vitro and in vivo evaluation of radiolabeled nanoparticles. Q J Nucl Med Mol Imaging 52:267–277
Singh B, Fu C, Bhattacharya J (2000) Vascular expression of the alpha(v)beta(3)-integrin in lung and other organs. Am J Physiol Lung Cell Mol Physiol 278:L217–L226
Terry SY, Abiraj K, Frielink C, van Dijk LK, Bussink J, Oyen WJ, Boerman OC (2014) Imaging integrin αvβ3 on blood vessels with 111In-RGD2 in head and neck tumor xenografts. J Nucl Med 55:281–286
Xie J, Huang J, Li X, Sun S, Chen X (2009) Iron oxide nanoparticle platform for biomedical applications. Curr Med Chem 16:1278–1294
Yang Y, Yu K, Zhang H, Dai J, Deng Z (2014) In vitro assessment of the dual-targeting behavior of a peptide-based magnetic resonance imaging contrast agent. Int J Mol Med 33:215–220
Zhang C, Jugold M, Woenne EC, Lammers T, Morgenstern B, Mueller MM, Zentgraf H, Bock M, Eisenhut M, Semmler W, Kiessling F (2007) Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res 67:1555–1562
Zhang F, Huang X, Zhu L, Guo N, Niu G, Swierczewska M, Lee S, Xu H, Wang AY, Mohamedali KA, Rosenblum MG, Lu G, Chen X (2012) Noninvasive monitoring of orthotopic glioblastoma therapy response using RGD-conjugated iron oxide nanoparticles. Biomaterials 33:5414–5422
Zheng SW, Huang M, Hong RY, Deng SM, Cheng LF, Gao B, Badami D (2014) RGD-conjugated iron oxide magnetic nanoparticles for magnetic resonance imaging contrast enhancement and hyperthermia. J Biomater Appl 28:1051–1059
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Deng, S., Zhang, W., Zhang, B. et al. Radiolabeled cyclic arginine-glycine-aspartic (RGD)-conjugated iron oxide nanoparticles as single-photon emission computed tomography (SPECT) and magnetic resonance imaging (MRI) dual-modality agents for imaging of breast cancer. J Nanopart Res 17, 19 (2015). https://doi.org/10.1007/s11051-014-2845-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2845-9