Abstract
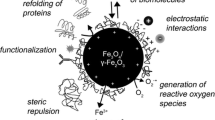
Magnetic iron oxide nanoparticles (IONP) are currently used for various neurobiological applications. To investigate the consequences of a treatment of brain cells with such particles, we have applied dimercaptosuccinate (DMSA)-coated IONP that had an average hydrodynamic diameter of 60 nm to oligodendroglial OLN-93 cells. After exposure to 4 mM iron applied as DMSA–IONP, these cells increased their total specific iron content within 8 h 600-fold from 7 to 4,200 nmol/mg cellular protein. The strong iron accumulation was accompanied by a change in cell morphology, although the cell viability was not compromized. DMSA–IONP treatment caused a concentration-dependent increase in the iron-dependent formation of reactive oxygen species and a decrease in the specific content of the cellular antioxidative tripeptide glutathione. During a 16 h recovery phase in IONP-free culture medium following exposure to DMSA–IONP, OLN-93 cells maintained their high iron content and replenished their cellular glutathione content. These data demonstrate that viable OLN-93 cells have a remarkable potential to deal successfully with the consequences of an accumulation of large amounts of iron after exposure to DMSA–IONP.







Similar content being viewed by others
Abbreviations
- AA:
-
Amino acids
- ANOVA:
-
Analysis of variance
- BSO:
-
Buthionine sulfoximine
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMSA:
-
Dimercaptosuccinate
- FCS:
-
Fetal calf serum
- GSH:
-
Glutathione
- GSSG:
-
Glutathione disulfide
- GSx:
-
Total glutathione
- IB:
-
Incubation buffer
- IONP:
-
Iron oxide nanoparticles
- LDH:
-
Lactate dehydrogenase
- PBS:
-
Phosphate buffered saline
- Phen:
-
Phenanthroline
- PI:
-
Propidium iodide
- ROS:
-
Reactive oxygen species
References
Abedini F, Hosseinkhani H, Ismail M, Chen YR, Omar AR, Chong PP, Domb AJ (2011) In vitro intracellular trafficking of biodegradable nanoparticles dextran-spermine in cancer cell lines. Int J Nanotechnol 8(8/9):712–723. doi:10.1504/IJNT.2011.04144
Apopa PL, Qian Y, Shao R, Guo NL, Schwegler-Berry D, Pacurari M, Porter D, Shi X, Vallyathan V, Castranova V, Flynn DC (2009) Iron oxide nanoparticles induce human microvascular endothelial cell permeability through reactive oxygen species production and microtubule remodeling. Part Fibre Toxicol 6:1. doi:10.1186/1743-8977-6-1
Baud O, Greene AE, Li J, Wang H, Volpe JJ, Rosenberg PA (2004) Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J Neurosci 24(7):1531–1540. doi:10.1523/JNEUROSCI.3989-03.2004
Benkovic SA, Connor JR (1993) Ferritin, transferrin, and iron in selected regions of the adult and aged rat brain. J Comp Neurol 338(1):97–113. doi:10.1002/cne.903380108
Berg JM, Ho S, Hwang W, Zebda R, Cummins K, Soriaga MP, Taylor R, Guo B, Sayes CM (2010) Internalization of carbon black and maghemite iron oxide nanoparticle mixtures leads to oxidant production. Chem Res Toxicol 23(12):1874–1882. doi:10.1021/tx100307h
Bhirde A, Xie J, Swierczewska M, Chen X (2011) Nanoparticles for cell labeling. Nanoscale 3(1):142–153. doi:10.1039/C0NR00493F
Bradl M, Lassmann H (2010) Oligodendrocytes: biology and pathology. Acta Neuropathol 119(1):37–53. doi:10.1007/s00401-009-0601-5
Buckinx R, Smolders I, Sahebali S, Janssen D, Smets I, Ameloot M, Rigo JM (2009) Morphological changes do not reflect biochemical and functional differentiation in OLN-93 oligodendroglial cells. J Neurosci Methods 184(1):1–9. doi:10.1016/j.jneumeth.2009.07.004
Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, Zywicke H, Miller B, van Gelderen P, Moskowitz BM, Duncan ID, Frank JA (2001) Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol 19(12):1141–1147. doi:10.1038/nbt1201-1141
Busch W, Bastian S, Trahorsch U, Iwe M, Kühnel D, Meißner T, Springer A, Gelinsky M, Richter V, Ikonomidou C (2011) Internalisation of engineered nanoparticles into mammalian cells in vitro: influence of cell type and particle properties. J Nanopart Res 13:293–310. doi:10.1007/s11051-010-0030-3
Buyukhatipoglu K, Clyne AM (2011) Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation. J Biomed Mater Res A 96(1):186–195. doi:10.1002/jbm.a.32972
Chen L, Mccrate JM, Lee JCM, Li H (2011) The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology 22(10):105708–105718. doi:10.1088/0957-4484/22/10/105708
Chertok B, Moffat BA, David AE, Yu F, Bergemann C, Ross BD, Yang VC (2008) Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials 29(4):487–496. doi:10.1016/j.biomaterials.2007.08.050
Connor JR, Menzies SL (1996) Relationship of iron to oligodendrocytes and myelination. Glia 17(2):83–93. doi:10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7
Ding J, Tao K, Li J, Song S, Sun K (2010) Cell-specific cytotoxicity of dextran-stabilized magnetite nanoparticles. Colloids Surf B Biointerfaces 79(1):184–190. doi:10.1016/j.colsurfb.2010.03.053
Dringen R, Hamprecht B (1996) Glutathione content as an indicator for the presence of metabolic pathways of amino acids in astroglial cultures. J Neurochem 67(4):1375–1382. doi:10.1046/j.1471-4159.1996.67041375.x
Dringen R, Hamprecht B (1998) Glutathione restoration as indicator for cellular metabolism of astroglial cells. Dev Neurosci 20(4–5):401–407. doi:10.1159/000017338
Dringen R, Kranich O, Hamprecht B (1997) The γ-glutamyl transpeptidase inhibitor acivicin preserves glutathione released by astroglial cells in culture. Neurochem Res 22(6):727–733. doi:10.1023/A:1027310328310
Dringen R, Kussmaul L, Hamprecht B (1998) Detoxification of exogenous hydrogen peroxide and organic hydroperoxides by cultured astroglial cells assessed by microtiter plate assay. Brain Res Brain Res Protoc 2(3):223–228. doi:10.1016/S1385-299X(97)00047-0
Dringen R, Pawlowski PG, Hirrlinger J (2005) Peroxide detoxification by brain cells. J Neurosci Res 79(1–2):157–165. doi:10.1002/jnr.20280
Dringen R, Koehler Y, Derr L, Tomba G, Schmidt MM, Treccani L, Colombi Ciacci L, Rezwan R (2011) Adsorption and reduction of glutathione disulfide on α-Al2O3 nanoparticles: experiments and modeling. Langmuir 27:9449–9457. doi:10.1021/la201856p
Fauconnier N, Pons JN, Roger J, Bee A (1997) Thiolation of maghemite nanoparticles by dimercaptosuccinic acid. J Colloid Interface Sci 194(2):427–433. doi:10.1006/jcis.1997.5125
Galaris D, Pantopoulos K (2008) Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit Rev Clin Lab Sci 45(1):1–23. doi:10.1080/10408360701713104
Ge Y, Zhang Y, Xia J, Ma M, He S, Nie F, Gu N (2009) Effect of surface charge and agglomerate degree of magnetic iron oxide nanoparticles on KB cellular uptake in vitro. Colloids Surf B Biointerfaces 73:294–301. doi:10.1016/j.colsurfb.2009.05.031
Geppert M, Hohnholt M, Gaetjen L, Grunwald I, Bäumer M, Dringen R (2009) Accumulation of iron oxide nanoparticles by cultured brain astrocytes. J Biomed Nanotechnol 5(3):285–293. doi:10.1166/jbn.2009.1033
Geppert M, Hohnholt MC, Thiel K, Nürnberger S, Grunwald I, Rezwan K, Dringen R (2011) Uptake of dimercaptosuccinate-coated magnetic iron oxide nanoparticles by cultured brain astrocytes. Nanotechnology 22(14):145101–145111. doi:10.1088/0957-4484/22/14/145101
Gubin SP (2009) Magnetic nanoparticles. Wiley-VCH, Weinheim
Hagens WI, Oomen AG, de Jong WH, Cassee FR, Sips AJ (2007) What do we (need to) know about the kinetic properties of nanoparticles in the body? Regul Toxicol Pharmacol 49(3):217–229. doi:10.1016/j.yrtph.2007.07.006
Hirrlinger J, Dringen R (2010) The cytosolic redox state of astrocytes: maintenance, regulation and functional implications for metabolite trafficking. Brain Res Rev 63(1–2):177–188. doi:10.1016/j.brainresrev.2009.10.003
Hirrlinger J, Resch A, Gutterer JM, Dringen R (2002a) Oligodendroglial cells in culture effectively dispose of exogenous hydrogen peroxide: comparison with cultured neurones, astroglial and microglial cells. J Neurochem 82(3):635–644. doi:10.1046/j.1471-4159.2002.00999.x
Hirrlinger J, Schulz JB, Dringen R (2002b) Glutathione release from cultured brain cells: multidrug resistance protein 1 mediates the release of GSH from rat astroglial cells. J Neurosci Res 69(3):318–326. doi:10.1002/jnr.10308
Hoepken HH, Korten T, Robinson SR, Dringen R (2004) Iron accumulation, iron-mediated toxicity and altered levels of ferritin and transferrin receptor in cultured astrocytes during incubation with ferric ammonium citrate. J Neurochem 88(5):1194–1202. doi:10.1046/j.1471-4159.2003.02236.x
Hohnholt M, Geppert M, Dringen R (2010a) Effects of iron chelators, iron salts, and iron oxide nanoparticles on the proliferation and the iron content of oligodendroglial OLN-93 cells. Neurochem Res 35(8):1259–1268. doi:10.1007/s11064-010-0184-5
Hohnholt MC, Geppert M, Nürnberger S, Von Byern J, Grunwald I, Dringen R (2010b) Advanced biomaterials accumulation of citrate-coated magnetic iron oxide nanoparticles by cultured brain astrocytes. Adv Eng Mater 12(12):B690–B694. doi:10.1002/adbi.201000055
Hohnholt MC, Geppert M, Dringen R (2011) Treatment with iron oxide nanoparticles induces ferritin synthesis but not oxidative stress in oligodendroglial cell. Acta Biomater (in press)
Jordan A, Scholz R, Maier-Hauff K, van Landeghem FK, Waldoefner N, Teichgraeber U, Pinkernelle J, Bruhn H, Neumann F, Thiesen B, von Deimling A, Felix R (2006) The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J Neurooncol 78(1):7–14. doi:10.1007/s11060-005-9059-z
Kabaso D, Gongadze E, Perutková S, Matschegewski C, Kralj-Iglic V, Beck U, van Rienen U, Iglic A (2011) Mechanics and electrostatics of the interactions between osteoblasts and titanium surface. Comput Methods Biomech Biomed Eng 14(5):469–482. doi:10.1080/10255842.2010.534986
Lamkowsky MC, Geppert M, Schmidt MM, Dringen R (2011) Magnetic field-induced acceleration of the accumulation of magnetic iron oxide nanoparticles by cultured brain astrocytes. J Biomed Mat Res A (in press)
Lewinski N, Colvin V, Drezek R (2008) Cytotoxicity of nanoparticles. Small 4(1):26–49. doi:10.1002/smll.200700595
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275
Mahmoudi M, Hosseinkhani H, Hosseinkhani M, Boutry S, Simchi A, Journeay WS, Subramani K, Laurent S (2011a) Magnetic resonance imaging tracking of stem cells in vivo using iron oxide nanoparticles as a tool for the advancement of clinical regenerative medicine. Chem Rev 111:253–280. doi:10.1021/cr1001832
Mahmoudi M, Stroeve P, Milani AS, Arbab AS (2011b) Superparamagnetic iron oxide nanoparticles: synthesis, surface engineering, cytotoxicity and biomedical applications. Nova Science Publishers, New York
McTigue DM, Tripathi RB (2008) The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem 107(1):1–19. doi:10.1111/j.1471-4159.2008.05570.x
Moller P, Jacobsen NR, Folkmann JK, Danielsen PH, Mikkelsen L, Hemmingsen JG, Vesterdal LK, Forchhammer L, Wallin H, Loft S (2010) Role of oxidative damage in toxicity of particulates. Free Radic Res 44(1):1–46. doi:10.3109/10715760903300691
Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311(5761):622–627. doi:10.1126/science.1114397
Pickard M, Chari D (2010) Enhancement of magnetic nanoparticle-mediated gene transfer to astrocytes by ‘magnetofection’: effects of static and oscillating fields. Nanomedicine (Lond) 5(2):217–232. doi:10.2217/nnm.09.109
Radu M, Munteanu MC, Petrache S, Serban AI, Dinu D, Hermenean A, Sima C, Dinischiotu A (2010) Depletion of intracellular glutathione and increased lipid peroxidation mediate cytotoxicity of hematite nanoparticles in MRC-5 cells. Acta Biochim Pol 57(3):355–360
Richardson DR, Baker E (1994) Two saturable mechanisms of iron uptake from transferrin in human melanoma cells: the effect of transferrin concentration, chelators, and metabolic probes on transferrin and iron uptake. J Cell Physiol 161(1):160–168. doi:10.1002/jcp.1041610119
Richter-Landsberg C, Heinrich M (1996) OLN-93: a new permanent oligodendroglia cell line derived from primary rat brain glial cultures. J Neurosci Res 45(2):161–173. doi:10.1002/(SICI)1097-4547(19960715)45:2<161:AID-JNR8>3.0.CO;2-8
Riemer J, Hoepken HH, Czerwinska H, Robinson SR, Dringen R (2004) Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal Biochem 331(2):370–375. doi:10.1016/j.ab.2004.03.049
Scheiber IF, Schmidt MM, Dringen R (2010) Zinc prevents the copper-induced damage of cultured astrocytes. Neurochem Int 57(3):314–322. doi:10.1016/j.neuint.2010.06.010
Schmidt MM, Dringen R (2011) GSH synthesis and metabolism. In: Gruetter R, Choi IY (eds) Advances in neurobiology, vol: neural metabolism in vivo. Springer, New York (in press)
Silva GA (2007) Nanotechnology approaches for drug and small molecule delivery across the blood brain barrier. Surg Neurol 67(2):113–116. doi:10.1016/j.surneu.2006.08.033
Soenen SJ, Nuytten N, De Meyer SF, De Smedt SC, De Cuyper M (2010) High intracellular iron oxide nanoparticle concentrations affect cellular cytoskeleton and focal adhesion kinase-mediated signaling. Small 6(7):832–842. doi:10.1002/smll.200902084
Soenen SJ, Himmelreich U, Nuytten N, De Cuyper M (2011) Cytotoxic effects of iron oxide nanoparticles and implications for safety in cell labelling. Biomaterials 32(1):195–205. doi:10.1016/j.biomaterials.2010.08.075
Subramani K, Hosseinkhani H, Khraisat A, Hosseinkhani M, Pathak Y (2009) Targeting nanoparticles as drug delivery systems for cancer treatment. Curr Nanosci 5(2):135–140. doi:10.2174/157341309788185406
Thiessen A, Schmidt MM, Dringen R (2010) Fumaric acid dialkyl esters deprive cultured rat oligodendroglial cells of glutathione and upregulate the expression of heme oxygenase 1. Neurosci Lett 475(1):56–60. doi:10.1016/j.neulet.2010.03.048
Valois CR, Braz JM, Nunes ES, Vinolo MA, Lima EC, Curi R, Kuebler WM, Azevedo RB (2010) The effect of DMSA-functionalized magnetic nanoparticles on transendothelial migration of monocytes in the murine lung via a β2 integrin-dependent pathway. Biomaterials 31(2):366–374. doi:10.1016/j.biomaterials.2009.09.053
Villanueva A, Canete M, Roca AG, Calero M, Veintemillas-Verdaguer S, Serna CJ, Morales Mdel P, Miranda R (2009) The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology 20:115103–115112. doi:10.1088/0957-4484/20/11/115103
Wang J, Chen Y, Chen B, Ding J, Xia G, Gao C, Cheng J, Jin N, Zhou Y, Li X, Tang M, Wang XM (2010) Pharmacokinetic parameters and tissue distribution of magnetic Fe3O4 nanoparticles in mice. Int J Nanomed 5:861–866. doi:10.2147/IJN.S13662
Weinstein JS, Varallyay CG, Dosa E, Gahramanov S, Hamilton B, Rooney WD, Muldoon LL, Neuwelt EA (2010) Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab 30(1):15–35. doi:10.1038/jcbfm.2009.192
Yang H (2010) Nanoparticle-mediated brain-specific drug delivery, imaging, and diagnosis. Pharm Res 27(9):1759–1771. doi:10.1007/s11095-010-0141-7
Yue ZG, Wei W, Lv PP, Yue H, Wang LY, Su ZG, Ma GH (2011) Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules 12(7):2440–2446. doi:10.1021/bm101482r
Acknowledgments
The authors would like to thank the Forschungsförderung of the University Bremen for financial support. Michaela C. Hohnholt is a member of the graduate school nanoToxCom. The authors would like to thank Mark Geppert (University of Bremen) for providing the DMSA-coated and citrate-coated IONP and Professor Christiane Richter-Landsberg (University of Oldenburg) for providing the OLN-93 cells.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hohnholt, M.C., Dringen, R. Iron-dependent formation of reactive oxygen species and glutathione depletion after accumulation of magnetic iron oxide nanoparticles by oligodendroglial cells. J Nanopart Res 13, 6761–6774 (2011). https://doi.org/10.1007/s11051-011-0585-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-011-0585-7