Abstract
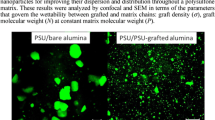
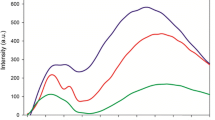
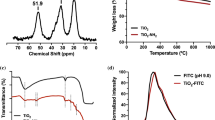
Gadolinium oxide nanoparticles are more and more used. They can notably provide interesting fluorescence properties. Herein they are incorporated into a non-aqueous-based polymer, the poly(methyl methacrylate). Their dispersion within the polymer matrix is the key to improve the composite properties. As-received gadolinium oxide nanopowders cannot be homogeneously dispersed in such a polymer matrix. Two surface treatments are, therefore, detailed and compared to achieve a good stability of the nanoparticles in a non-aqueous solvent such as the 2-butanone. Then, once the liquid suspensions have been stabilized, they are used to prepare nanocomposites with homogeneous particles dispersion. The two approaches proposed are an hybrid approach based on the growth of a silica shell around the gadolinium oxide nanoparticles, and followed by a suitable silane functionalization; and a non-hybrid approach based on the use of surfactants. The surface treatments and formulations involved in both methods are detailed, adjusted and compared. Thanks to optical methods and in particular to the use of a ‘home made’ confocal microscope, the dispersion homogeneity within the polymer can be assessed. Both methods provide promising and conclusive results.









Similar content being viewed by others
References
Daniel M-C, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104(1):293–346
El-Toni AM, Yin S et al (2005) Coating of calcia-doped ceria with amorphous silica shell by seeded polymerization technique. Mater Res Bull 40(7):1059–1064
Etienne S, Becker C et al (2007) Effects of incorporation of modified silica nanoparticles on the mechanical and thermal properties of PMMA. J Therm Anal Calorim 87(1):101–104
Feng J, Shan G et al (2003) Functionalized europium oxide nanoparticles used as a fluorescent label in an immunoassay for atrazine. Anal Chem 75(19):5282–5286
Goldburt ET, Kulkarni B et al (1997) Size dependent efficiency in Tb doped Y2O3 nanocrystalline phosphor. J Lumin 72–74:190–192
Goubard F, Vidal F et al (2007) Synthesis and luminescent properties of PEO/lanthanide oxide nanoparticle hybrid films. J Lumin 126(2):289–296
Iijima M, Tsukada M et al (2007) Effect of surface interaction of silica nanoparticles modified by silane coupling agents on viscosity of methylethylketone suspension. J Colloid Interface Sci 305(2):315–323
Jódar-Reyes AB, Ortega-Vinuesa JL et al (2006a) Electrokinetic behavior and colloidal stability of polystyrene latex coated with ionic surfactants. J Colloid Interface Sci 297(1):170–181
Jódar-Reyes AB, Martín-Rodríguez A et al (2006b) Effect of the ionic surfactant concentration on the stabilization/destabilization of polystyrene colloidal particles. J Colloid Interface Sci 298(1):248–257
Konstantinos C, Konstantinos MP et al (2007) Thermal and dynamic mechanical behaviour of bionanocomposites: Fumed silica nanoparticles dispersed in poly(vinyl pyrrolidone), chitosan, and poly(vinyl alcohol). J Appl Polym Sci 110(3):1739–1749
Lee C-S, Lee J-S et al (2003) Dispersion control of Fe2O3 nanoparticles using a mixed type of mechanical and ultrasonic milling. Mater Lett 57(18):2643–2646
Lee D-W, Kim N-H et al (2005) Effect of nonionic surfactants on the stability of alumina slurry for Cu CMP. Mater Sci Eng B 118(1–3):293–300
Louis C, Bazzi R et al (2005) Nanosized hybrid particles with double luminescence for biological labeling. Chem Mater 17(7):1673–1682
Mine E, Yamada A et al (2003) Direct coating of gold nanoparticles with silica by a seeded polymerization technique. J Colloid Interface Sci 264(2):385–390
Natuvetty PR (1999) Aspects of pigment dispersion—a review. Paintindia 49(6):53–58
Palla BJ, Shah DO (2002) Stabilization of high ionic strength slurries using surfactant mixtures: molecular factors that determine optimal stability. J Colloid Interface Sci 256(1):143–152
Plaza RC, Gómez-Lopera SA et al (2001) Magnetic properties of composite hematite/yttrium oxide colloidal particles. J Colloid Interface Sci 240(1):48–53
Samuel J, Raccurt O, Poncelet O, Auger A, Ling W-L, Cherns P, Grunwald D, Tillement O (2010) Surface characterizations of fluorescent functionalized silica nanoparticles: from the macroscale to the nanoscale. J Nanopart Res 12(6):2255
Sharma PK, Jilavi MH et al (2002) Influence of initial pH on the particle size and fluorescence properties of the nano scale Eu(III) doped yttria. J Phys Chem Solids 63(1):171–177
Shea L, McKittrick J, Lopez O (1996) Synthesis of red-emitting, small particle size luminescent oxides using an optimized combustion process. J Am Ceram Soc 79:3257–3265
Tseng WJ, Tzeng F (2006) Effect of ammonium polyacrylate on dispersion and rheology of aqueous ITO nanoparticle colloids. Colloids Surfaces A 276(1–3):34–39
Wang S-F, Hsu Y-F et al (2005) Silica coating on ultrafine [alpha]-alumina particles. Mater Sci Eng A 395(1–2):148–152
Weichold O, Dederichs T et al (2007) The dispersion-stability diagram of boehmite nanoparticles in aqueous AOT solutions. J Colloid Interface Sci 306(2):300–306
Zhang W, Zhang Y et al (2002) Study on preparation and optic properties of nano europium oxide-ethanol sol by pulsed laser ablation. Thin Solid Films 417(1–2):43–46
Acknowledgments
The authors would like to thank the ‘NanOpTec’ center (Lyon, France) for access to the optical spectroscopy and the imaging confocal microscopy facilities. They also would like to thank Carol Grossiord and the team of Science et Surface (Ecully, France) for the XPS analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samuel, J., Raccurt, O., Mancini, C. et al. Homogeneous dispersion of gadolinium oxide nanoparticles into a non-aqueous-based polymer by two surface treatments. J Nanopart Res 13, 2417–2428 (2011). https://doi.org/10.1007/s11051-010-0129-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-010-0129-6