Abstract
Background
Pancreatic β-cells are susceptible to oxidative stress, leading to β-cell death and dysfunction due to enhanced ROS levels and type 2 diabetes. To inhibit the β-cells damages induced by the oxidative stress, the present study investigates the beneficial effect of various peptides (WL15, RF13, RW20, IW13 and MF18) of immune related proteins (cysteine and glycine-rich protein 2, histone acetyltransferase, vacuolar protein sorting associated protein 26B, serine threonine-protein kinase and CxxC zinc finger protein, respectively). Also, the molecular mechanism of WL15 from cysteine and glycine-rich protein 2 on β-cell regeneration was identified through PEPCK and insulin pathway.
Materials and methods
In this study, a total of five peptides including WL15, RF13, RW20, IW13, and MF18 were derived from immune-related proteins such as cysteine and glycine-rich protein 2, histone acetyltransferase, vacuolar protein sorting associated protein 26B, serine threonine-protein kinase and CxxC zinc finger protein, respectively. These protein sequences were obtained from an earlier constructed transcriptome database of a teleost Channa striatus. The identified peptides were evaluated for their antioxidant as well as antidiabetic activity. Based on the in silico analysis and in-vitro screening experiments, WL15 was predicted to have better antioxidant and antidiabetic activity among the five different peptides. Therefore, WL15 alone was further analyzed for apoptosis, antioxidant capacity, glucose metabolism, and gene expression performance, which was investigated on the alloxan (500 µM) induced zebrafish in vivo larval model.
Results
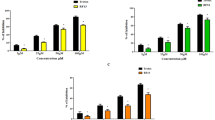
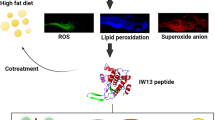
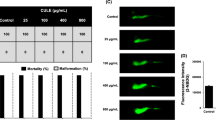
The results showed alloxan exposure to zebrafish larvae for a day, the ROS was generated in the β-cells. Interestingly, WL15 treatment showed a protective effect by reducing the toxicity of alloxan exposed zebrafish larvae by increasing their survival and heart rate. Moreover, WL15 reduced the intracellular ROS level and apoptosis in alloxan-induced larvae. The superoxide anion and lipid peroxidation levels are also reduced by improving the glutathione content after the WL15 treatment. Besides, WL15 treatment increased the proliferation rate of β-cells and decreased the glucose level. Further, the gene expression studies revealed that WL15 treatment normalized the PEPCK expression while upregulating the insulin expression in alloxan exposed larvae.
Conclusion
Overall, the findings indicate that WL15 of cysteine and glycine-rich protein 2 can act as a potential antioxidant for type 2 diabetes patients in respect of improving β-cell regeneration.






Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Code availability
Not applicable.
Change history
06 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11033-023-08365-w
Abbreviations
- AGEs:
-
Advanced glycation end products
- PEPCK:
-
Phosphoenolpyruvate carboxykinase
- DHE:
-
Dihydroethidium
- NDA:
-
NDA, naphthalene-2,3-dicarboxal-dehyde
- T2D:
-
Type 2 diabetes
- ROS:
-
Reactive oxygen species
- DCFDA:
-
2'-7'-Dichlorofluorescein diacetate
- DPPP:
-
Diphenyl-1-pyrenylphosphine
- SD:
-
Standard deviation
- EDTA:
-
Ethylenediaminetetraacetic acid
- 2-NBDG:
-
2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose
- DCFDA:
-
2',7'-Dichlorodihydrofluorescein diacetate
- CSRP2:
-
Cysteine and glycine-rich protein 2
- HATs:
-
Histone acetyltransferase
- VPS26B:
-
Vacuolar protein sorting associated protein 26B
- STPK:
-
Serine threonine-protein kinase
References
Cerf ME (2013) Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne) 4:1–12. https://doi.org/10.3389/fendo.2013.00037
Saisho Y (2015) β-cell dysfunction: its critical role in prevention and management of type 2 diabetes. World J Diabetes 6:109. https://doi.org/10.4239/wjd.v6.i1.109
Gurgul-Convey E, Mehmeti I, Plötz T et al (2016) Sensitivity profile of the human EndoC-βH1 beta cell line to proinflammatory cytokines. Diabetologia 59:2125–2133. https://doi.org/10.1007/s00125-016-4060-y
Drews G, Krippeit-Drews P, Duïfer M (2010) Oxidative stress and beta-cell dysfunction. Pflugers Arch Eur J Physiol 460:703–718. https://doi.org/10.1007/s00424-010-0862-9
Eguchi N, Vaziri ND, Dafoe DC, Ichii H (2021) The role of oxidative stress in pancreatic β cell dysfunction in diabetes. Int J Mol Sci 22:1–18. https://doi.org/10.3390/ijms22041509
Stancill JS, Broniowska KA, Oleson BJ et al (2019) Pancreatic -cells detoxify H2O2 through the peroxiredoxin/thioredoxin antioxidant system. J Biol Chem 294:4843–4853. https://doi.org/10.1074/jbc.RA118.006219
Matschinsky FM (1996) A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes 45:223–241. https://doi.org/10.2337/diab.45.2.223
Moss LG, Caplan TV, Moss JB (2013) Imaging beta cell regeneration and interactions with islet vasculature in transparent adult zebrafish. Zebrafish 10:249–257. https://doi.org/10.1089/zeb.2012.0813
Zang L, Maddison LA, Chen W (2018) Zebrafish as a model for obesity and diabetes. Front Cell Dev Biol 6:1–13. https://doi.org/10.3389/fcell.2018.00091
Guru A, Issac PK, Saraswathi NT et al (2021) Deteriorating insulin resistance due to WL15 peptide from cysteine and glycine-rich protein 2 in high glucose-induced rat skeletal muscle L6 cells. Cell Biol Int 45:1698–1709. https://doi.org/10.1002/cbin.11608
Mohd Shafri MA, Abdul Manan MJ (2012) Therapeutic potential of the haruan (Channa striatus): from food to medicinal uses. Malays J Nutr 18:125–136
Wang J, Wang H (2017) Oxidative stress in pancreatic beta cell regeneration. Oxid Med Cell Longev. https://doi.org/10.1155/2017/1930261
Yu Z, Wu S, Zhao W et al (2018) Identification and the molecular mechanism of a novel myosin-derived ACE inhibitory peptide. Food Funct 9:364–370. https://doi.org/10.1039/c7fo01558e
Pearman NA, Ronander E, Smith AM, Morris GA (2020) The identification and characterisation of novel bioactive peptides derived from porcine liver. Curr Res Food Sci 3:314–321. https://doi.org/10.1016/j.crfs.2020.11.002
Cakır B, Okuyan B, Sener G, Tunali-Akbay T (2021) Investigation of beta-lactoglobulin derived bioactive peptides against SARS-CoV-2 (COVID-19): in silico analysis. Eur J Pharmacol 891:173781. https://doi.org/10.1016/j.ejphar.2020.173781
Velayutham M, Guru A, Gatasheh MK et al (2022) Molecular docking of SA11, RF13 and DI14 peptides from vacuolar protein sorting associated protein 26B against cancer proteins and in vitro investigation of its anticancer potency in Hep-2 cells. Int J Pept Res Ther. https://doi.org/10.1007/s10989-022-10395-0
Sangeetha R, Vedasree N (2012) In vitro α -amylase inhibitory activity of the leaves of thespesia populnea. ISRN Pharmacol 2012:1–4. https://doi.org/10.5402/2012/515634
Manjunathan T, Guru A, Arokiaraj J, Gopinath P (2021) 6-gingerol and semisynthetic 6-gingerdione counteract oxidative stress induced by ROS in zebrafish. Chem Biodivers. https://doi.org/10.1002/cbdv.202100650
Guru A, Lite C, Freddy AJ et al (2021) Intracellular ROS scavenging and antioxidant regulation of WL15 from cysteine and glycine-rich protein 2 demonstrated in zebrafish in vivo model. Dev Comp Immunol 114:103863. https://doi.org/10.1016/j.dci.2020.103863
Issac PK, Lite C, Guru A et al (2021) Tryptophan-tagged peptide from serine threonine-protein kinase of Channa striatus improves antioxidant defence in L6 myotubes and attenuates caspase 3–dependent apoptotic response in zebrafish larvae. Fish Physiol Biochem 47:293–311. https://doi.org/10.1007/s10695-020-00912-7
Sudhakaran G, Prathap P, Guru A et al (2022) Anti-inflammatory role demonstrated both in vitro and in vivo models using non-steroidal tetranortriterpenoid, Nimbin (N1) and its analogues (N2 and N3) that alleviate the domestication of alternative medicine. Cell Biol Int 24:327–332. https://doi.org/10.1002/cbin.11769
Velayutham M, Ojha B, Issac PK et al (2021) NV14 from serine O-acetyltransferase of cyanobacteria influences the antioxidant enzymes in vitro cells, gene expression against H2O2 and other responses in vivo zebrafish larval model. Cell Biol Int 45:2331–2346. https://doi.org/10.1002/cbin.11680
Sarkar P, Guru A, Raju SV et al (2021) GP13, an Arthrospira platensis cysteine desulfurase-derived peptide, suppresses oxidative stress and reduces apoptosis in human leucocytes and zebrafish (Danio rerio) embryo via attenuated caspase-3 expression. J King Saud Univ - Sci 33:101665. https://doi.org/10.1016/j.jksus.2021.101665
Sudhakaran G, Prathap P, Guru A et al (2022) Reverse pharmacology of Nimbin-N2 attenuates alcoholic liver injury and promotes the hepatoprotective dual role of improving lipid metabolism and downregulating the levels of inflammatory cytokines in zebrafish larval model. Mol Cell Biochem. https://doi.org/10.1007/s11010-022-04448-7
Haridevamuthu B, Manjunathan T, Guru A (2022) Amelioration of acrylamide induced neurotoxicity by benzo [b ] thiophene analogs via glutathione redox dynamics in zebrafish larvae. Brain Res 1788:147941. https://doi.org/10.1016/j.brainres.2022.147941
Lite C, Guru A, Juliet MJ, Arockiaraj J (2022) Embryonic exposure to butylparaben and propylparaben induced developmental toxicity and triggered anxiety-like neurobehavioral response associated with oxidative stress and apoptosis in the head of zebrafish larvae. Environ Toxicol. https://doi.org/10.1002/tox.23545
Li Y, Li X, Chu Q et al (2020) Russula alutacea Fr. polysaccharide ameliorates inflammation in both RAW264.7 and zebrafish (Danio rerio) larvae. Int J Biol Macromol 145:740–749. https://doi.org/10.1016/j.ijbiomac.2019.12.218
Lee J, Jung DW, Kim WH et al (2013) Development of a highly visual, simple, and rapid test for the discovery of novel insulin mimetics in living vertebrates. ACS Chem Biol 8:1803–1814. https://doi.org/10.1021/cb4000162
Velayutham M, Guru A, Arasu MV et al (2021) GR15 peptide of S-adenosylmethionine synthase (SAMe) from Arthrospira platensis demonstrated antioxidant mechanism against H2O2 induced oxidative stress in in-vitro MDCK cells and in vivo zebrafish larvae model. J Biotechnol 342:79–91. https://doi.org/10.1016/j.jbiotec.2021.10.010
Guru A, Sudhakaran G, Velayutham M et al (2022) Daidzein normalized gentamicin-induced nephrotoxicity and associated pro-inflammatory cytokines in MDCK and zebrafish: possible mechanism of nephroprotection. Comp Biochem Physiol Part C Toxicol Pharmacol 258:109364. https://doi.org/10.1016/j.cbpc.2022.109364
Haridevamuthu B, Manjunathan T, Guru A, Saravana R (2022) Hydroxyl containing benzo[b]thiophene analogs mitigates the acrylamide induced oxidative stress in the zebrafish larvae by stabilizing the glutathione redox cycle. Life Sci. https://doi.org/10.1016/j.lfs.2022.120507
Ghassem M, Arihara K, Babji AS et al (2011) Purification and identification of ACE inhibitory peptides from Haruan (Channa striatus) myofibrillar protein hydrolysate using HPLC-ESI-TOF MS/MS. Food Chem 129:1770–1777. https://doi.org/10.1016/j.foodchem.2011.06.051
Guru A, Velayutham M, Arockiaraj J (2022) Lipid-lowering and antioxidant activity of RF13 peptide from vacuolar protein sorting-associated protein 26B (VPS26B) by modulating lipid metabolism and oxidative stress in HFD induced obesity in zebrafish larvae. Int J Pept Res Ther 28:74. https://doi.org/10.1007/s10989-022-10376-3
Prabha N, Guru A, Harikrishnan R et al (2022) Neuroprotective and antioxidant capability of RW20 peptide from histone acetyltransferases caused by oxidative stress-induced neurotoxicity in in vivo zebrafish larval model. J King Saud Univ - Sci 100:101861. https://doi.org/10.1016/j.jksus.2022.101861
Nagaram P, Pasupuleti M, Arockiaraj J (2020) CxxC zinc finger protein derived peptide, MF18 functions against biofilm formation. Protein J 39:337–349. https://doi.org/10.1007/s10930-020-09904-1
Pavithra K, Vadivukkarasi S (2015) Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima (Jacq.) Cogn. Food Sci Hum Wellness 4:42–46. https://doi.org/10.1016/j.fshw.2015.02.001
Lenzen S, Drinkgern J, Tiedge M (1996) Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20:463–466. https://doi.org/10.1016/0891-5849(96)02051-5
Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2002) Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23:599–622. https://doi.org/10.1210/er.2001-0039
Ramkumar KM, Lee AS, Krishnamurthi K et al (2009) Gymnema montanum H. protects against alloxan-induced oxidative stress and apoptosis in pancreatic β-cells. Cell Physiol Biochem 24:429–440. https://doi.org/10.1159/000257480
Vahdatpour T, Nokhodchi A, Zakeri-Milani P et al (2019) Leucine–glycine and carnosine dipeptides prevent diabetes induced by multiple low-doses of streptozotocin in an experimental model of adult mice. J Diabetes Investig 10:1177–1188. https://doi.org/10.1111/jdi.13018
Moriya S, Yokoyama H, Fukuda M et al (2000) Glutathione depletion enhances the formation of superoxide anion released into hepatic sinusoids after lipopolysaccharide challenge. Alcohol Clin Exp Res 24:59–63. https://doi.org/10.1111/j.1530-0277.2000.tb00014.x
Nam YH, Hong BN, Rodriguez I et al (2015) Synergistic potentials of coffee on injured pancreatic islets and insulin action via KATP channel blocking in zebrafish. J Agric Food Chem 63:5612–5621. https://doi.org/10.1021/acs.jafc.5b00027
Kwon SJ, Hwang SJ, Jung Y et al (2017) A synthetic Nitraria alkaloid, isonitramine protects pancreatic β-cell and attenuates post-prandial hyperglycemia. Metabolism 70:107–115. https://doi.org/10.1016/j.metabol.2017.02.002
Acknowledgements
The authors extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/191), King Saud University, Riyadh, Saudi Arabia.
Funding
Researchers Supporting Project Number (RSP-2021/191), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
AG and JA contributed to the concept and design of the study; AG and GS performed the experiments; MHA. BOA. AJ and JA contributed significantly to resources, data analysis, manuscript preparation and perform the analysis with constructive discussions; JA supervised and checked the manuscript; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This research does not involve any human objects; however, we have performed few assays using zebrafish embryo and larvae. The fish were handled and experimented carefully as per the Institute Animal Handling Procedure and Ethical Approval and Clearence (No. SAF/IAEC/211215/004).
Consent to participate
All the authors listed in the manuscript have approved the manuscript.
Consent for publication
The data provided in the manuscript is approved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The affiliation of the author "Annie Juliet" is corrected as "Foundation for Aquaculture Innovations and Technology Transfer (FAITT), Thoraipakkam, Chennai 600 097, Tamil Nadu, India.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guru, A., Sudhakaran, G., Almutairi, M.H. et al. β-cells regeneration by WL15 of cysteine and glycine-rich protein 2 which reduces alloxan induced β-cell dysfunction and oxidative stress through phosphoenolpyruvate carboxykinase and insulin pathway in zebrafish in-vivo larval model. Mol Biol Rep 49, 11867–11879 (2022). https://doi.org/10.1007/s11033-022-07882-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07882-4