Abstract
Background
Turkey is one of the major exporters of mandarins in the Mediterranean region. Seedlessness in citrus, which is one of the most desired fruit quality traits, especially in fresh mandarin export markets, can be obtained via triploidy as in many fruit species. Triploid plants can be recovered by 2x × 2x hybridizations in citrus, as well as 2x × 4x and 4x × 2x crosses. Hence, we aimed to develop local triploid hybrids by using the embryo rescue technique in five crosses using eight different citrus varieties in the present study.
Methods and results
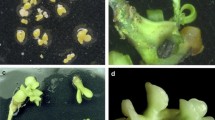
Embryos isolated from abortive seeds derived by 135 days after pollinations were cultured on modified Murashige and Tucker (MT) medium by adding different levels of GA3 to achieve a high germination rate. A population of 574 plants was developed as a result of embryo rescue. After screening the ploidy levels of this 574-plant population with the aid of flow cytometry, 4 triploids from ‘Encore’ × ‘Murcott’, 8 triploids from ‘Fortune’ × ‘Willow leaf’, 1 triploid from ‘Kiyomi’ × ‘Murcott’, and 1 triploid from ‘Ortanique’ × ‘Murcott’ hybridization were recovered. Triploid hybrid plants and related parents were analyzed with SSR markers heterozygotic for parental mandarin varieties. In addition, we evaluated stomatal characteristics of diploid and triploid hybrids obtained from different crosses. Stomatal traits of diploid and triploid hybrids in all crosses significantly differed except the stomata index.
Conclusions
Genotyping of triploid plants confirmed using Simple Sequence Repeat (SSR) molecular markers and five SSRs were able to identify three alleles of triploid hybrids. Selected triploid mandarin hybrids have been grafted on several rootstocks for field trials and are in the process of yield and quality performances.



Similar content being viewed by others
References
FAO (2020) FAOSTAT. http://www.fao.org/faostat/en/#data/QC. Accessed 8 Jan 2020
Jain SM, Brar DS, Ahloowalia BS (1998) Somaclonal variation and induced mutations in crop improvement. Springer, Netherlands
Ollitrault P, Dambier D, Luro F, Froelicher Y (2008) Ploidy manipulation for breeding seedless triploid citrus. In: Janick J (ed) Plant breeding reviews. Wiley, Hoboken, pp 323–352
Aleza P, Juárez J, Cuenca J et al (2010) Recovery of citrus triploid hybrids by embryo rescue and flow cytometry from 2x × 2x sexual hybridisation and its application to extensive breeding programs. Plant Cell Rep 29:1023–1034. https://doi.org/10.1007/s00299-010-0888-7
Geraci G, Esen A, Soost RK (1975) Triploid progenies of citrus cultivars from 2x × 2x crosses. J Hered 66:177–178. https://doi.org/10.1093/oxfordjournals.jhered.a108607
Aleza P, Juárez J, Ollitrault P, Navarro L (2009) Production of tetraploid plants of non apomictic citrus genotypes. Plant Cell Rep 28:1837–1846. https://doi.org/10.1007/s00299-009-0783-2
Carimi F, de Pasquale F, Puglia AM (1998) In vitro rescue of zygotic embryos of sour orange, Citrus aurantium L., and their detection based on RFLP analysis. Plant Breed 117:261–266. https://doi.org/10.1111/j.1439-0523.1998.tb01936.x
Yildiz E, Kaplankiran M, Demirkeser TH et al (2013) Identification of zygotic and nucellar ındividuals produced from several citrus crosses using SSRs markers. Not Bot Hort Agrobot Cluj 41:478. https://doi.org/10.15835/nbha4129037
Ollitrault-Sammarcelli F, Legave JM, Michaux-Ferriere N, Hirsch AM (1994) Use of flow cytometry for rapid determination of ploidy level in the genus Actinidia. Sci Hortic 57:303–313. https://doi.org/10.1016/S0304-4238(94)90113-9
Ollitrault P, Dambier D, Jacquemond C et al (1996) In vitro rescue and selection of spontaneous triploids by flow cytometry for easy peeler citrus breeding
Bohanec B (2003) Ploidy determination using flow cytometry. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants: a manual. Springer, Dordrecht, pp 397–403
Ochatt SJ (2008) Flow cytometry in plant breeding. Cytometry A 73A:581–598. https://doi.org/10.1002/cyto.a.20562
Navarro L, Juárez J, Aleza P, Pina JA (2003) Recovery of triploid seedless mandarin hybrids from 2n × 2n and 2n × 4n crosses by embryo rescue and flow cytometry. In: Vasil IK (ed) Plant Biotechnology 2002 and beyond. Springer, Dordrecht, pp 541–544
Froelicher Y, Bouffin J, Dambier D, Ollitrault P (2012) Triploid seedless mandarin breeding in France: S02O02. In: XII International Citrus Congress: Book of abstract. https://agritrop.cirad.fr/567275/. Accessed 18 Feb 2020
García Lidón A (2003) La selección clonal de limonero “fino”: Incidencia del patrón. Aspectos morfológicos, agronómicos y bioquímicos. http://purl.org/dc/dcmitype/Text. Universidad Politécnica de Cartagena
Pérez-Tornero O, Porras I (2008) Assessment of polyembryony in lemon: rescue and in vitro culture of immature embryos. Plant Cell Tissue Organ Cult 93:173–180. https://doi.org/10.1007/s11240-008-9358-0
Murashige T, Tucker DH (1969) Growth factor requirements of Citrus tissue culture. Proc First Int Citrus Sympos 3:1155–1160
Beaulieu JM, Leitch IJ, Patel SU et al (2008) Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol 179:975–986. https://doi.org/10.1111/j.1469-8137.2008.02528.x
Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19:1349
Kaçar YA, Simsek O, Solmaz I et al (2012) Genetic diversity among melon accessions (Cucumis melo) from Turkey based on SSR markers. Genet Mol Res 11:4622–4631. https://doi.org/10.4238/2012.November.29.2
Kepiro JL, Roose ML (2007) Nucellar embryony. In: Khan IA (ed) Citrus genetics, breeding and biotechnology. CABI, Wallingford, pp 141–149
Soost RK, Roose ML (1996) Citrus. In: Fruit breeding: tree and tropical fruits. Wiley, pp 257–323
Esen A, Soost RK (1971) Unexpected triploids in citrus: their origin, identification, and possible use. J Hered 62:329–333. https://doi.org/10.1093/oxfordjournals.jhered.a108186
Guo WW, Liang WJ, Xie KD et al (2016) Exploitation of polyploids from 39 citrus seedling populations. Acta Hortic 1135:11–16. https://doi.org/10.17660/ActaHortic.2016.1135.2
Vardi A, Levin I, Carmi N (2008) Induction of seedlessness in citrus: from classical techniques to emerging biotechnological approaches. J Am Soc Hortic Sci 133:117–126. https://doi.org/10.21273/JASHS.133.1.117
Carimi F (2005) Somatic embryogenesis protocol: citrus. In: Jain SM, Gupta PK (eds) Protocol for somatic embryogenesis in woody plants. Springer, Berlin, pp 321–343
Das A, Paul AK, Chaudhuri SK (2000) Micropropagation of sweet orange, Citrus sinensis Osbeck. For the development of nucellar seedlings. Indian J Exp Biol
Wakana A, Ngo BX, Iwamasa M (2004) Germinability of embryos during seed development in Citrus (Rutaceae). J Faculty Agric Kyushu Univ 49:49–59
Jaskani MJ, Khan IA, Khan MM (2005) Fruit set, seed development and embryo germination in interploid crosses of citrus. Sci Hortic 107:51–57. https://doi.org/10.1016/j.scienta.2005.06.011
Kurt S, Ulger S (2014) Production of common sour orange × carrizo citrange hybrids using embryo rescue. Int J Fruit Sci 14:42–48. https://doi.org/10.1080/15538362.2013.801729
Chagas EA, Pasqual M, Ramos JD et al (2003) Development of globular embryos from the hybridization between “Pêra Rio” sweet orange and “Poncã” mandarin. Rev Bras Frutic 25:483–488. https://doi.org/10.1590/S0100-29452003000300031
Jumin HB, Nito N (1996) Plant regeneration via somatic embryogenesis from protoplasts of six plant species related to Citrus. Plant Cell Rep 15:332–336. https://doi.org/10.1007/BF00232366
Gmitter FG, Ling XB, Deng XX (1990) Induction of triploid Citrus plants from endosperm calli in vitro. Theoret Appl Genet 80:785–790. https://doi.org/10.1007/BF00224192
Loureiro J, Pinto G, Lopes T et al (2005) Assessment of ploidy stability of the somatic embryogenesis process in Quercus suber L. using flow cytometry. Planta 221:815–822. https://doi.org/10.1007/s00425-005-1492-x
Guerra D, Schifino-Wittmann MT, Schwarz SF et al (2016) Tetraploidization in citrus rootstocks: effect of genetic constitution and environment in chromosome duplication. Crop Breed Appl Biotechnol 16:35–41. https://doi.org/10.1590/1984-70332016v16n1a6
Navarro L, Aleza P, Cuenca J et al (2015) The mandarın trıploıd breedıng program ın Spaın. Acta Hortic. https://doi.org/10.17660/ActaHortic.2015.1065.48
Aryavand A, Ehdaie B, Tran B, Waines JG (2003) Stomatal frequency and size differentiate ploidy levels in Aegilops neglecta. Genet Resour Crop Evol 50:175–182. https://doi.org/10.1023/A:1022941532372
Usman M, Fatima B, Gillani KA et al (2008) Exploitation of potential target tissues to develop polyploids in citrus. Pak J Bot 40:1755–1766
Beck SL, Visser G, Dunlop RW, Hare PD (2005) A comparison of direct (flow cytometry) and indirect (stomatal guard cell lengths and chloroplast numbers) techniques as a measure of ploidy in black wattle, Acacia mearnsii (de Wild). S Afr J Bot 71:354–358. https://doi.org/10.1016/S0254-6299(15)30109-5
Bastianel M, Schwarz SF, Coleta Filho HD et al (1998) Identification of zygotic and nucellar tangerine seedlings (Citrus spp.) using RAPD. Genet Mol Biol. https://doi.org/10.1590/S1415-47571998000100020
Ruiz C, Paz Breto M, Asíns MJ (2000) A quick methodology to identify sexual seedlings in citrus breeding programs using SSR markers. Euphytica 112:89–94. https://doi.org/10.1023/A:1003992719598
Carlos de Oliveira A, Novac Garcia A, Cristofani M, Machado MA (2002) Identification of citrus hybrids through the combination of leaf apex morphology and SSR markers. Euphytica 128:397–403. https://doi.org/10.1023/A:1021223309212
Andrade-Rodríguez M, Villegas-Monter A, Carrillo-Castañeda G, García-Velázquez A (2004) Polyembryony and identification of Volkamerian lemon zygotic and nucellar seedlings using RAPD. Pesq Agropec Bras 39:551–559. https://doi.org/10.1590/S0100-204X2004000600006
Tan M, Song J, Deng X (2007) Production of two mandarin × trifoliate orange hybrid populations via embryo rescue with verification by SSR analysis. Euphytica 157:155–160. https://doi.org/10.1007/s10681-007-9407-5
Golein B, Fifaei R, Ghasemi M (2011) Identification of zygotic and nucellar seedlings in citrus interspecific crosses by inter simple sequence repeats (ISSR) markers. Afr J Biotechnol 10:18965–18970. https://doi.org/10.5897/AJB11.2332
Fatima B, Usman M, Khan MS et al (2015) Identification of citrus polyploids using chromoseme counts, morphological and SSR markers. Pak J Agric Sci 52:107–114
Kijas JMH, Thomas MR, Fowler JCS, Roose ML (1997) Integration of trinucleotide microsatellites into a linkage map of Citrus. Theor Appl Genet 94:701–706. https://doi.org/10.1007/s001220050468
Ferrante SP, Lucretti S, Reale S et al (2010) Assessment of the origin of new citrus tetraploid hybrids (2n = 4x) by means of SSR markers and PCR based dosage effects. Euphytica 173:223–233. https://doi.org/10.1007/s10681-009-0093-3
Luro F, Maddy F, Ollitrault P, Rist D (2000) Identification of 2n gametes parental origin and mode of nuclear restitution of spontaneous triploid citrus hybrids. In: 9th ISC congress, 3–7 December 2000, Orlando (Etats-Unis). https://agritrop.cirad.fr/477422/. Accessed 4 Mar 2020
Acknowledgements
The authors are grateful that the manuscript has been read and critically corrected by Dr. Ben FABER who works at the Division of Agriculture and Natural Resources, University of California Cooperative Extension. The authors also would like to thank the ‘Atlas Tarim’ and ‘Incesulu Tarim’ companies for supplying parental plant materials used in hybridizations.
Funding
This research was funded by the Scientific Research Projects Coordination Unit of Cukurova University (Grant Number FBA-2018-11126).
Author information
Authors and Affiliations
Contributions
BC and TY performed hybridization studies and in vitro embryo culture, DD and YAK performed in SSR screening and result interpretation. BC and SE helped in data analysis and preparation of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflicts of interest or competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cimen, B., Yesiloglu, T., Donmez, D. et al. Recovering triploid citrus hybrids from 2x × 2x sexual crosses with the aid of embryo rescue and flow cytometry in Turkey. Mol Biol Rep 49, 5625–5634 (2022). https://doi.org/10.1007/s11033-022-07555-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07555-2