Abstract
Background
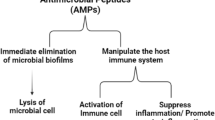
Current approaches used to overcome microbial infections are becoming inefficient due to the overuse or misuse of antibiotics. Antimicrobial peptides (AMPs) are one of the most promising substitutional candidates for commercial antibiotics.
Methods and Results
The publications in the field of in silico design of AMPs with focus on the wound-healing AMPs were searched though SCOPUS and PubMed. Through publications, it was reported that a number of AMPs approved for clinical use have also showed efficient wound-healing activity. Todays, the design and production of synthetic types of AMPs have attracted attention in order to expand their applications and to cope with their limitations and adverse effects. In this paper, the currently published researches in the field of AMPs and their wound-healing features were summarized and the strategies used in AMPs design and development have been reviewed. Moreover, different databases and online servers used in this regard were summarized.
Conclusion
Design and development of active AMPs, especially those with wound-healing activity, can be performed using bioinformatics servers and software, regarding AMPs structure-activity relationships.




Similar content being viewed by others
References
Magana M et al (2020) The value of antimicrobial peptides in the age of resistance. The Lancet Infectious Diseases
Patrulea V et al (2019) Chitosan-based systems for controlled delivery of antimicrobial peptides for biomedical application. Functional Chitosan. Springer, pp 415–455
Ting DSJ et al (2020) Strategies in translating the therapeutic potentials of host defense peptides. Front Immunol 11:983
Oliveira NG et al (2020) Physicochemical-guided design of cathelicidin-derived peptides generates membrane active variants with therapeutic potential. Sci Rep 10(1):1–11
Chen CH, Lu TK (2020) Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 9(1):24
Puentes PR et al (2020) Design, Screening, and Testing of Non-Rational Peptide Libraries with Antimicrobial Activity: In Silico and Experimental Approaches. Antibiotics 9(12):854
Patrulea V, Borchard G, Jordan O (2020) An update on antimicrobial peptides (AMPs) and their delivery strategies for wound infections. Pharmaceutics 12(9):840
Koo HB, Seo J (2019) Antimicrobial peptides under clinical investigation. Pept Sci 111(5):e24122
Mahlapuu M et al (2016) Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol 6:194
Akhmetova A, Heinz A (2021) Electrospinning Proteins for Wound Healing Purposes: Opportunities and Challenges. Pharmaceutics 13(1):4
Malik E et al (2016) pH dependent antimicrobial peptides and proteins, their mechanisms of action and potential as therapeutic agents. Pharmaceuticals 9(4):67
Melo MN, Ferre R, Castanho MA (2009) Antimicrobial peptides: linking partition, activity and high membrane-bound concentrations. Nat Rev Microbiol 7(3):245–250
de Fernández M et al (2020) Antifungal peptides as therapeutic agents. Front Cell Infect Microbiol 10:105
Cardoso MH et al (2020) Computer-aided design of antimicrobial peptides: are we generating effective drug candidates? Front Microbiol 10:3097
Neff JA et al (2020) Novel Antimicrobial Peptides Formulated in Chitosan Matrices are Effective Against Biofilms of Multidrug-Resistant Wound Pathogens. Mil Med 185(Supplement1):637–643
Liu S et al (2018) Novel 3D structure based model for activity prediction and design of antimicrobial peptides. Sci Rep 8(1):1–12
Lee EY et al (2017) What can machine learning do for antimicrobial peptides, and what can antimicrobial peptides do for machine learning? Interface focus 7(6):20160153
Mookherjee N et al (2020) Antimicrobial host defence peptides: Functions and clinical potential. Nat Rev Drug Discovery 19(5):311–332
Boparai JK, Sharma PK (2020) Mini review on antimicrobial peptides, sources, mechanism and recent applications. Protein Pept Lett 27(1):4–16
Xie Z et al (2019) The Analogs of Temporin-GHa Exhibit a Broader Spectrum of Antimicrobial Activity and a Stronger Antibiofilm Potential against Staphylococcus aureus. Molecules 24(22):4173
Mishra AK et al (2018) Tryptophan-rich and proline-rich antimicrobial peptides. Molecules 23(4):815
Koehbach J, Craik DJ (2019) The vast structural diversity of antimicrobial peptides. Trends Pharmacol Sci 40(7):517–528
Strieker M, Tanović A, Marahiel MA (2010) Nonribosomal peptide synthetases: structures and dynamics. Curr Opin Struct Biol 20(2):234–240
Hughes P et al (2000) The cytotoxic plant protein, β-purothionin, forms ion channels in lipid membranes. J Biol Chem 275(2):823–827
Li J et al (2021) Plant antimicrobial peptides: structures, functions, and applications. Bot Stud 62(1):1–15
Hultmark D et al (1982) Insect immunity: isolation and structure of cecropin D and four minor antibacterial components from Cecropia pupae. Eur J Biochem 127(1):207–217
Cao J et al (2018) Yeast-based synthetic biology platform for antimicrobial peptide production. ACS Synth Biol 7(3):896–902
Ponnappan N et al (2015) Membrane-active peptides from marine organisms—antimicrobials, cell-penetrating peptides and peptide toxins: applications and prospects. Probiotics and antimicrobial proteins 7(1):75–89
Tan J et al (2020) Synthetic macromolecules as therapeutics that overcome resistance in cancer and microbial infection. Biomaterials 252:120078
Wuersching SN et al (2021) Inhibitory effect of LL-37 and human lactoferricin on growth and biofilm formation of anaerobes associated with oral diseases. Anaerobe 67:102301
Yang B et al (2020) Significance of LL-37 on Immunomodulation and Disease Outcome. BioMed Research International, 2020: p. 8349712
Ikonomova SP et al (2020) Effects of histatin 5 modifications on antifungal activity and kinetics of proteolysis. Protein Sci 29(2):480–493
Reis Zambom C, Henrique da F, Fonseca, Santesso Garrido S (2020) Bio- and Nanotechnology as the Key for Clinical Application of Salivary Peptide Histatin: A Necessary Advance. Microorganisms 8(7):1024
Brice DC, Diamond G (2020) Antiviral Activities of Human Host Defense Peptides. Curr Med Chem 27(9):1420–1443
Rodriguez C et al (2020) Antimicrobial Secretions of Toads (Anura, Bufonidae): Bioactive Extracts and Isolated Compounds against Human Pathogens. Antibiotics 9(12):843
McMillan KAM, Coombs MRP (2020) Review: Examining the Natural Role of Amphibian Antimicrobial Peptide Magainin Molecules 25(22):5436
Yi H-Y et al (2014) Insect antimicrobial peptides and their applications. Appl Microbiol Biotechnol 98(13):5807–5822
Buonocore F et al (2021) Attacins: A Promising Class of Insect Antimicrobial Peptides. Antibiotics 10(2):212
Slavokhotova AA et al (2017) Hevein-like antimicrobial peptides of plants. Biochem (Moscow) 82(13):1659–1674
Afroz M et al (2020) Ethnobotany and Antimicrobial Peptides From Plants of the Solanaceae Family: An Update and Future Prospects. Front Pharmacol 11:565
Anderssen EL et al (1998) Antagonistic activity of Lactobacillus plantarum C11: two new two-peptide bacteriocins, plantaricins EF and JK, and the induction factor plantaricin A. Appl Environ Microbiol 64(6):2269–2272
Pirtskhalava M et al (2021) DBAASP v3: database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Res 49(D1):D288–D297
Semreen MH et al (2018) Recent updates of marine antimicrobial peptides. Saudi Pharm J 26(3):396–409
Huang H-N et al (2017) Antimicrobial peptide Epinecidin-1 promotes complete skin regeneration of methicillin-resistant Staphylococcus aureus-infected burn wounds in a swine model. Oncotarget 8(13):21067–21080
Chung EMC et al (2017) Komodo dragon-inspired synthetic peptide DRGN-1 promotes wound-healing of a mixed-biofilm infected wound. npj Biofilms and Microbiomes 3(1):9
Di Somma A et al (2020) Antimicrobial and antibiofilm peptides. Biomolecules 10(4):652
Ho Y-H et al (2016) Systematic analysis of intracellular-targeting antimicrobial peptides, bactenecin 7, hybrid of pleurocidin and dermaseptin, proline–arginine-rich peptide, and lactoferricin B, by using Escherichia coli proteome microarrays. Mol Cell Proteomics 15(6):1837–1847
Barreto-Santamaría A et al (2016) A new synthetic peptide having two target of antibacterial action in E. coli ML35. Frontiers in microbiology, 7: p. 2006
Wimley WC (2010) Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol 5(10):905–917
Rowe-Magnus DA et al (2019)Cathelicidin peptides restrict bacterial growth via membrane perturbation and induction of reactive oxygen species. MBio, 10(5)
Graf M, Wilson DN (2019) Intracellular antimicrobial peptides targeting the protein synthesis machinery. Antimicrobial Peptides, Advances in Experimental Medicine and Biology. Springer, Singapore, pp 73–89
Rončević T, Puizina J, Tossi A (2019) Antimicrobial peptides as anti-infective agents in pre-post-antibiotic era? Int J Mol Sci 20(22):5713
Marchand C et al (2006) Covalent binding of the natural antimicrobial peptide indolicidin to DNA abasic sites. Nucleic Acids Res 34(18):5157–5165
Falanga A et al (2017) Cyclic peptides as novel therapeutic microbicides: Engineering of human defensin mimetics. Molecules 22(7):1217
van den Bergen G et al (2020) Curved or linear? Predicting the 3-dimensional structure of α‐helical antimicrobial peptides in an amphipathic environment. FEBS Lett 594(6):1062–1080
Bradshow J (2003) Cationic antimicrobial peptides. Issues for potential clinical use. Biodrugs 17:233–240
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55(1):27–55
Lee T-H, Hall KN, Aguilar M-I (2016) Antimicrobial peptide structure and mechanism of action: a focus on the role of membrane structure. Curr Top Med Chem 16(1):25–39
Greco I et al (2020) Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci Rep 10(1):13206
Yount NY et al (2006) Advances in antimicrobial peptide immunobiology. Pept Science: Original Res Biomolecules 84(5):435–458
Wang G (2020) Bioinformatic analysis of 1000 amphibian antimicrobial peptides uncovers multiple length-dependent correlations for peptide design and prediction. Antibiotics 9(8):491
Uematsu N, Matsuzaki K (2000) Polar Angle as a Determinant of Amphipathic α-Helix-Lipid Interactions: A Model Peptide Study. Biophys J 79(4):2075–2083
Al Tall Y et al (2019) Design and characterization of a new hybrid peptide from LL-37 and BMAP-27. Infection and drug resistance. 12:1035–1045
Waghu FH et al (2018) Designing antibacterial peptides with enhanced killing kinetics. Front Microbiol 9:325
Torres MD et al (2019) Peptide design principles for antimicrobial applications. J Mol Biol 431(18):3547–3567
Houyvet B et al (2018) Design of antimicrobial peptides from a cuttlefish database. Amino Acids 50(11):1573–1582
Wang G (2015) 18 - Database Resources Dedicated to Antimicrobial Peptides. Antimicrobial Resistance and Food Safety. Academic Press, San Diego, pp 365–384. C.-Y. Chen, X. Yan, and C.R. Jackson, Editors
Waghu FH, Idicula-Thomas S (2020) Collection of antimicrobial peptides database and its derivatives: Applications and beyond. Protein Sci 29(1):36–42
Wang G, Li X, Wang Z (2016) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Research, 44(D1): p. D1087-D1093
Kang X et al (2019) DRAMP 2.0, an updated data repository of antimicrobial peptides. Sci Data 6(1):148
Ramos-Martín F et al (2019) ADAPTABLE: a comprehensive web platform of antimicrobial peptides tailored to the user’s research. Life Sci alliance 2(6):e201900512
Piotto SP et al (2012) YADAMP: yet another database of antimicrobial peptides. Int J Antimicrob Agents 39(4):346–351
Usmani SS et al (2018) AntiTbPdb: a knowledgebase of anti-tubercular peptides. Database, 2018
Di Luca M et al (2015) BaAMPs: the database of biofilm-active antimicrobial peptides. Biofouling 31(2):193–199
Whitmore L, Wallace BA (2004) The Peptaibol Database: a database for sequences and structures of naturally occurring peptaibols. Nucleic Acids Res 32(Database issue):D593–D594
Yang J, Roy A, Zhang Y (2013) BioLiP: a semi-manually curated database for biologically relevant ligand-protein interactions. Nucleic Acids Res 41(Database issue):D1096–D1103
Wang CK et al (2008) CyBase: a database of cyclic protein sequences and structures, with applications in protein discovery and engineering. Nucleic Acids Res 36(Database issue):D206–D210
Novković M et al (2012) DADP: the database of anuran defense peptides. Bioinformatics 28(10):1406–1407
Flissi A et al (2020) Norine: update of the nonribosomal peptide resource. Nucleic Acids Res 48(D1):D465–d469
Tyagi A et al (2015) CancerPPD: a database of anticancer peptides and proteins. Nucleic Acids Res 43(Database issue):D837–D843
Chen W et al (2016) iACP: a sequence-based tool for identifying anticancer peptides. Oncotarget 7(13):16895–16909
Gautam A et al (2014) Hemolytik: a database of experimentally determined hemolytic and non-hemolytic peptides. Nucleic Acids Res 42(Database issue):D444–D449
Agrawal P et al (2016) CPPsite 2.0: a repository of experimentally validated cell-penetrating peptides. Nucleic Acids Res 44(D1):D1098–D1103
Khatun MS, Hasan MM, Kurata H (2019) PreAIP: Computational Prediction of Anti-inflammatory Peptides by Integrating Multiple Complementary Features. Front Genet 10:129–129
Gasteiger E et al (2005) Protein identification and analysis tools on the ExPASy server. : p. 571–607
Gautam A et al (2015) Computer-Aided Virtual Screening and Designing of Cell-Penetrating Peptides. Methods Mol Biol 1324:59–69
Atanaki FF et al (2020) BIPEP: Sequence-based Prediction of Biofilm Inhibitory Peptides Using a Combination of NMR and Physicochemical Descriptors. ACS Omega 5:7290–7297
Chaudhary K et al (2016) A Web Server and Mobile App for Computing Hemolytic Potency of Peptides. Sci Rep 6(1):22843
Gupta S et al (2013) In silico approach for predicting toxicity of peptides and proteins. PLoS ONE 8(9):e73957
Sharma A et al (2014) Designing of peptides with desired half-life in intestine-like environment. BMC Bioinformatics 15(1):282
Meher PK et al (2017) Predicting antimicrobial peptides with improved accuracy by incorporating the compositional, physico-chemical and structural features into Chou’s general PseAAC. Sci Rep 7:42362
Molero-Abraham M et al (2015) EPIPOX: Immunoinformatic Characterization of the Shared T-Cell Epitome between Variola Virus and Related Pathogenic Orthopoxviruses. J Immunol Res 2015:738020
Lamiable A et al (2016) PEP-FOLD3: faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res 44(W1):W449–W454
Gautier R et al (2008) HELIQUEST: a web server to screen sequences with specific alpha-helical properties. Bioinformatics 24(18):2101–2102
Behrendt R, White P, Offer J (2016) Advances in Fmoc solid-phase peptide synthesis. J Pept Sci 22(1):4–27
Azari M, Asad S, Mehrnia MR (2020) Heterologous production of porcine derived antimicrobial peptide PR-39 in Escherichia coli using SUMO and intein fusion systems. Protein expression 169:105568
Chen Q-c et al (2021) High-level expression and purification of melittin in Escherichia coli using SUMO fusion partner. Int J Pept Res 27(1):9–15
Wang Y-Q, Cai J-Y (2007) High-level expression of acidic partner-mediated antimicrobial peptide from tandem genes inEscherichia coli. Appl Biochem Biotechnol 141(2):203–213
Roca-Pinilla R et al (2020) A new generation of recombinant polypeptides combines multiple protein domains for effective antimicrobial activity. Microb Cell Fact 19(1):1–7
Veiga A et al (2019) Colorimetric microdilution assay: Validation of a standard method for determination of MIC, IC50%, and IC90% of antimicrobial compounds. J Microbiol Methods 162:50–61
O’Toole GA Microtiter dish biofilm formation assay. Journal of visualized experiments: JoVE, 2011(47): p.2437
Song Y et al (2019) A short peptide potentially promotes the healing of skin wound.Bioscience Reports, 39(3)
Souza PF et al (2020) Synthetic antimicrobial peptides: From choice of the best sequences to action mechanisms. Biochimie 175:132–145
Gomes A et al (2017) Wound-healing peptides for treatment of chronic diabetic foot ulcers and other infected skin injuries. Molecules 22(10):1743
Hirsch T et al (2009) Human beta-defensin‐3 promotes wound healing in infected diabetic wounds. J Gene Med 11(3):220–228
Kolar SS, McDermott AM (2011) Role of host-defence peptides in eye diseases. Cell Mol Life Sci 68(13):2201–2213
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there is no conflict of interest.
Compliance with Ethical Standards
This article does not contain any studies with animals performed by any of the authors and also, does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fathi, F., Ghobeh, M. & Tabarzad, M. Anti-Microbial Peptides: Strategies of Design and Development and Their Promising Wound-Healing Activities. Mol Biol Rep 49, 9001–9012 (2022). https://doi.org/10.1007/s11033-022-07405-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07405-1