Abstract
Background
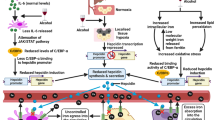
Non-alcoholic fatty liver disease (NAFLD) is a multifactorial disorder with complicated pathophysiology. Trimethylamine-N-oxide (TMAO) has been thought to be correlated with the pathogenesis of NAFLD. The single nucleotide polymorphisms (SNPs) of hepatic flavin-containing monooxygenase 3 (FMO3) regulate the concentration of TMAO. This case-control study investigated the plasma levels of TMAO as well as its possible correlation with the frequency of specific genotype of FMO3 (-2650C>G, -2543T>A, -2177G>C, -2589C>T, -2106G>A polymorphisms) in Kurdish patients with NAFLD.
Methods and Results
In 85 confirmed NAFLD patients and 30 healthy individuals, triglycerides (TG), total cholesterol (Chol), low-density lipoprotein (LDL), high-density lipoprotein (HDL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) activities were measured. TMAO was also measured using the LC–MS/MS method. High-resolution melting analysis was applied to determine FMO3 genotypes. Plasma TMAO levels were significantly higher in patients (p = 0.030). A CC genotype with a frequency of 12.9% for SNP -2177G>C was found in Kurdish NAFLD patients. The distribution of the GC genotype was also significantly different (p = 0.017).
Conclusions
The current results provide documentation for high circulatory levels of TMAO and its possible correlation with the presence of the specific genotype -2177G>C FMO3 in Kurdish NAFLD patients.




Similar content being viewed by others
References
Arrese M et al (2016) Innate immunity and inflammation in NAFLD/NASH. Digest Dis Sci 61:1294–13035
Wree A et al (2013) From NAFLD to NASH to cirrhosis—new insights into disease mechanisms. Nat reviews Gastroenterol Hepatol 10(11):627–636
Ono M, Okamoto N, Saibara T (2010) The latest idea in NAFLD/NASH pathogenesis. Clin J Gastroenterol 3(6):263–270
Tiniakos DG, Vos MB, Brunt EM (2010) Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol 5:145–171
Vernon RG (2005) Lipid metabolism during lactation: a review of adipose tissue-liver interactions and the development of fatty liver. J Dairy Res 72(4):460
Leung C et al (2016) The role of the gut microbiota in NAFLD. Nat Reviews Gastroenterol Hepatol 13(7):412–425
Wang Z et al (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472(7341):57–63
Rath S et al (2019) Potential TMA-producing bacteria are ubiquitously found in mammalia. Front Microbiol 10:2966
Sun X et al (2016) Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun 481(1–2):63–70
Randrianarisoa E et al (2016) Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci Rep 6(1):1–9
Huijbers MM et al (2014) Flavin dependent monooxygenases. Arch Biochem Biophys 544:2–17
Reddy RR et al (2010) Characterization of human flavin-containing monooxygenase (FMO) 3 and FMO5 expressed as maltose-binding protein fusions. Drug Metab Dispos 38(12):2239–2245
Koukouritaki SB et al (2005) Discovery of novel flavin-containing monooxygenase 3 (FMO3) single nucleotide polymorphisms and functional analysis of upstream haplotype variants. Mol Pharmacol 68(2):383–392
Shimizu M et al (2014) Relationships between flavin-containing mono‐oxygenase 3 (FMO3) genotype and trimethylaminuria phenotype in a J apanese population. Br J Clin Pharmacol 77(5):839–851
Chen Y-m et al (2016) Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep 6(1):1–9
Pan JJ, Fallon MB (2014) Gender and racial differences in nonalcoholic fatty liver disease. World J Hepatol 6(5):274–283
Lankarani KB et al (2013) Non alcoholic fatty liver disease in southern Iran: a population based study. Hepat Mon 13(5):e9248
Balakrishnan M et al (2021) Women have lower risk of nonalcoholic fatty liver disease but higher risk of progression vs men: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 19(1):61–71e15
Akhavan Rezayat A et al (2018) Association between smoking and non-alcoholic fatty liver disease: a systematic review and meta-analysis. SAGE Open Med 6:2050312117745223
Okamoto M et al (2018) Cigarette smoking is a risk factor for the onset of fatty liver disease in nondrinkers: A longitudinal cohort study. PLoS ONE 13(4):e0195147
Charatcharoenwitthaya P, Karaketklang K, Aekplakorn W (2020) Cigarette Smoking Increased Risk of Overall Mortality in Patients With Non-alcoholic Fatty Liver Disease: A Nationwide Population-Based Cohort Study. Front Med (Lausanne) 7:604919
Rutledge SM, Asgharpour A (2020) Smoking and liver disease. Gastroenterol Hepatol (N Y) 16(12):617–625
Azzalini L et al (2010) Cigarette smoking exacerbates nonalcoholic fatty liver disease in obese rats. Hepatology 51(5):1567–1576
Ponciano-Rodriguez G, Mendez-Sanchez N (2010) Cigarette smoking and fatty liver. Ann Hepatol 9(2):215–218
Mitchell SC, Smith RL (2001) Trimethylaminuria: the fish malodor syndrome. Drug Metab Dispos 29(4 Pt 2):517–521
Lang DH et al (1998) Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: selective catalysis by FMO3. Biochem Pharmacol 56(8):1005–1012
Yousuf A, McVey DG, Ye S (2022) Relationship between red meat metabolite trimethylamine N-oxide and cardiovascular disease.
Shi C et al (2022) Changes of flavin-containing monooxygenases and trimethylamine-N-oxide may be involved in the promotion of non-alcoholic fatty liver disease by intestinal microbiota metabolite trimethylamine. Biochem Biophys Res Commun
Liu X et al (2017) Preoperative serum TMAO level is a new prognostic marker for colorectal cancer. Biomark Med 11(5):443–447
Missailidis C et al (2016) Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS ONE 11(1):e0141738
Delzenne NM, Cani PD (2011) Gut microbiota and the pathogenesis of insulin resistance. Curr Diab Rep 11(3):154–159
Croyal M et al (2020) Plasma trimethylamine N-oxide and risk of cardiovascular events in patients with type 2 diabetes. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgaa188
Xu R, Wang Q (2016) Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst Biol 10(Suppl 3):63
Zhang AQ, Mitchell SC, Smith RL (1999) Dietary precursors of trimethylamine in man: a pilot study. Food Chem Toxicol 37(5):515–520
Ding L et al (2018) Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis 17(1):1–8
Chen M-l et al (2016) Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio. https://doi.org/10.1128/mBio.02210-15
Shih DM et al (2015) Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res 56(1):22–37
Seldin MM et al (2016) Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappaB. J Am Heart Assoc. https://doi.org/10.1128/mBio.02210-15
Ganz M, Szabo G (2013) Immune and inflammatory pathways in NASH. Hep Intl 7(2):771–781
Li Z et al (2019) Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest 99(3):346–357
Acknowledgements
This work, as a MSc student’s thesis, was supported financially by vice chancellor in research of Kurdistan University of Medical Sciences [Grant Numbers 1397/237-97.10.23]. This study was entirely performed at the Tohid hospital and laboratories of Kurdistan University of Medical Sciences. We thank strategic technologies laboratory network of Iran and the centres of Cellular and Molecular Research and Liver& Digestive Research of Kurdistan University of Medical Sciences for their valuable assistance. We also thank all those who helped us as well as all patients and healthy subjects participated in this research.
Funding
This study was conducted as a dissertation of a master’s student in clinical biochemistry. It was supported financially by vice chancellor in research of Kurdistan University of Medical Sciences [grant numbers 1397/237 − 97.10.23].
Author information
Authors and Affiliations
Contributions
Mohammad Moradzad carried out the sample collection, all laboratory works, and final report preparation. Mohammad Abdi assisted with HRM analysis and manuscript preparation. Farshad Sheikh Esmaeili as a gastroenterologist helped to introduce and confirm NAFLD patients and healthy individuals. Dana Ghaderi helped with sample collection. Khaled Rahmani provided assistance for statistical analysis. Mohammad Raman Moloudi helped as an advisor. Zakaria Vahabzadeh carried out the design, supervised the study, and prepared the manuscript. The manuscript’s contents have been read and approved by all authors. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Medical Ethics Committee of Kurdistan University of Medical Science.and with the 1964 Helsinki declaration. All subjects entered the study with full knowledge of the research project and gave informed written consent to participate. Ethics were fully observed in all protocols. The study protocol was approved by the Medical Ethics Committee of Kurdistan University of Medical Science under the code IR.MUK.REC.1397/237.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moradzad, M., Abdi, M., Sheikh Esmaeili, F. et al. Possible correlation between high circulatory levels of trimethylamine-N-oxide and 2177G>C polymorphisms of hepatic flavin containing monooxygenase 3 in Kurdish Population with non-alcoholic fatty liver disease. Mol Biol Rep 49, 5927–5937 (2022). https://doi.org/10.1007/s11033-022-07375-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07375-4