Abstract
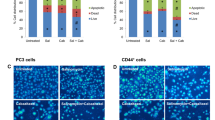
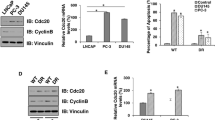
One of the major barriers in cancer therapy is the resistance to conventional therapies and cancer stem cells (CSCs) are among the main causes of this problem. CD133 as a CSC marker displays stem cell-like properties, tumorigenic capacity, and drug resistance in various cancers. However, the molecular mechanism behind CD133 function in prostate cancer (PC) still remains unclear. This research aimed to illustrate the probabilistic mechanism of CD133-siRNA and paclitaxel in the reduction of chemoresistance in PC cells. To measure the cell viability, migratory capacity, CSCs properties, invasive potential, apoptosis and cell cycle progression of the cells, the MTT, wound healing, spheroid assay, colony formation assay, DAPI staining and flow cytometry assays were applied in the LNCaP cell line, respectively. Also, quantitative real-time PCR (qRT-PCR) and western blot method were used for measuring the expression of CD133 and the effects of CD133 silencing on the AKT/mTOR/c-myc axis and pro-metastatic genes expression. We showed that the CD133-siRNA considerably decreased the CD133 expression. Moreover, CD133-siRNA and paclitaxel treatment significantly decreased cell proliferation and also inhibited the ability of cell migration and invasion and reduced pro-metastatic genes expression. Additionally, we found that the simultaneous use of CD133-siRNA and paclitaxel increased the paclitaxel‐induced apoptosis. Our results confirmed that CD133 silencing combined with paclitaxel synergistically could suppress cell migration, invasion, and proliferation and enhance the chemosensitivity compared with mono treatment. Therefore, CD133 silencing therapy could be viewed as a promising and efficient strategy in PC targeted therapies.








Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Divrik RT, Türkeri L, Şahin AF et al (2012) Prediction of response to androgen deprivation therapy and castration resistance in primary metastatic prostate cancer. Urol Int 88:25–33. https://doi.org/10.1159/000334539
Erdogan S, Turkekul K, Dibirdik I et al (2019) Midkine silencing enhances the anti–prostate cancer stem cell activity of the flavone apigenin: cooperation on signaling pathways regulated by ERK, p38, PTEN, PARP, and NF-κB. Invest New Drugs. https://doi.org/10.1007/s10637-019-00774-8
Ni J, Cozzi P, Hao J et al (2014) Cancer stem cells in prostate cancer chemoresistance. Curr Cancer Drug Targets 14:225–240. https://doi.org/10.2174/1568009614666140328152459
Shiozawa Y, Berry JE, Eber MR et al (2016) The marrow niche controls the cancer stem cell phenotype of disseminated prostate cancer. Oncotarget 7:41217–41232. https://doi.org/10.18632/oncotarget.9251
Harris KS, Kerr BA (2017) Prostate cancer stem cell markers drive progression, therapeutic resistance, and bone metastasis. Stem Cells Int. https://doi.org/10.1155/2017/8629234
Aghajani M, Mansoori B, Mohammadi A et al (2019) New emerging roles of CD133 in cancer stem cell: Signaling pathway and miRNA regulation. J Cell Physiol 234:21642–21661. https://doi.org/10.1002/jcp.28824
Chang HH, Chen BY, Wu CY et al (2011) Hedgehog overexpression leads to the formation of prostate cancer stem cells with metastatic property irrespective of androgen receptor expression in the mouse model. J Biomed Sci 18:6. https://doi.org/10.1186/1423-0127-18-6
Jang JW, Song Y, Kim SH et al (2017) CD133 confers cancer stem-like cell properties by stabilizing EGFR-AKT signaling in hepatocellular carcinoma. Cancer Lett 389:1–10. https://doi.org/10.1016/j.canlet.2016.12.023
Burnett JC, Rossi JJ, Tiemann K (2011) Current progress of siRNA/shRNA therapeutics in clinical trials. Biotechnol J 6:1130–1146. https://doi.org/10.1002/biot.201100054
Fire AZ (2007) Gene silencing by double-stranded RNA (Nobel Lecture). Angew Chemie Int Ed 46:6966–6984. https://doi.org/10.1002/anie.200701979
Mansoori B, Mohammadi A, Goldar S et al (2016) Silencing of high mobility group isoform I-C (HMGI-C) enhances paclitaxel chemosensitivity in breast adenocarcinoma cells (MDAMB-468). Adv Pharm Bull 6:171–177. https://doi.org/10.15171/apb.2016.024
Mansoori B, Shotorbani SS, Baradaran B (2014) RNA interference and its role in cancer therapy. Adv Pharm Bull 4:313–321. https://doi.org/10.5681/apb.2014.046
Shajari N, Mansoori B, Davudian S et al (2017) Overcoming the challenges of siRNA delivery: nanoparticle strategies. Curr Drug Deliv 14:36–46. https://doi.org/10.2174/1567201813666160816105408
Ojo D, Lin X, Wong N et al (2015) Prostate cancer stem-like cells contribute to the development of castration-resistant prostate cancer. Cancers (Basel) 7:2290–2308. https://doi.org/10.3390/cancers7040890
Collins AT, Berry PA, Hyde C et al (2005) Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 65:10946–10951. https://doi.org/10.1158/0008-5472.CAN-05-2018
Maitland NJ, Collins A (2005) A tumour stem cell hypothesis for the origins of prostate cancer. BJU Int 96:1219–1223. https://doi.org/10.1111/j.1464-410X.2005.05744.x
Vander Griend DJ, Karthaus WL, Dalrymple S et al (2008) The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res 68:9703–9711. https://doi.org/10.1158/0008-5472.CAN-08-3084
Glumac PM, LeBeau AM (2018) The role of CD133 in cancer: a concise review. Clin Transl Med 7:1–14. https://doi.org/10.1186/s40169-018-0198-1
Richardson GD, Robson CN, Lang SH et al (2004) CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci 117:3539–3545. https://doi.org/10.1242/jcs.01222
Reyes EE, Kunovac SK, Duggan R et al (2013) Growth kinetics of CD133-positive prostate cancer cells. Prostate 73:724–733. https://doi.org/10.1002/pros.22616
Chou T-C, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55. https://doi.org/10.1016/0065-2571(84)90007-4
Reyes EE, Gillard M, Duggan R, et al (2015) Molecular analysis of CD133-positive circulating tumor cells from patients with metastatic castration-resistant prostate cancer. J Transl Sci 1:https://oatext.com/Molecular-analysis-of-CD133-posi
Reyes EE, Vander Weele DJ, Isikbay M et al (2014) Quantitative characterization of androgen receptor protein expression and cellular localization in circulating tumor cells from patients with metastatic castration-resistant prostate cancer. J Transl Med 12:1–15. https://doi.org/10.1186/s12967-014-0313-z
Angelastro JM, Lame MW (2010) Overexpression of CD133 promotes drug resistance in C6 glioma cells. Mol Cancer Res 8:1105–1115. https://doi.org/10.1158/1541-7786.MCR-09-0383
Li K, Li X, Tian J et al (2016) Downregulation of DNA-PKcs suppresses P-gp expression via inhibition of the Akt/NF-κB pathway in CD133-positive osteosarcoma MG-63 cells. Oncol Rep 36:1973–1980. https://doi.org/10.3892/or.2016.4991
El-Khattouti A, Selimovic D, Haïkel Y et al (2014) Identification and analysis of CD133+ melanoma stem-like cells conferring resistance to taxol: An insight into the mechanisms of their resistance and response. Cancer Lett 343:123–133. https://doi.org/10.1016/j.canlet.2013.09.024
Zhu Y, Yu J, Wang S et al (2014) Overexpression of CD133 enhances chemoresistance to 5-fluorouracil by activating the PI3K/Akt/p70S6K pathway in gastric cancer cells. Oncol Rep 32:2437–2444. https://doi.org/10.3892/or.2014.3488
Song S, Pei G, Du Y et al (2018) Interaction between CD133 and PI3K-p85 promotes chemoresistance in gastric cancer cells. Am J Transl Res 10:304–314
Rappa G, Fodstad O, Lorico A (2008) The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells 26:3008–3017. https://doi.org/10.1634/stemcells.2008-0601
Elsaba TMA, Martinez-Pomares L, Robins AR et al (2010) The stem cell marker CD133 associates with enhanced colony formation and cell motility in colorectal cancer. PLoS ONE. https://doi.org/10.1371/journal.pone.0010714
Horst D, Scheel SK, Liebmann S et al (2009) The cancer stem cell marker CD133 has high prognostic impact but unknown functional relevance for the metastasis of human colon cancer. J Pathol 219:427–434. https://doi.org/10.1002/path.2597
Morgia G, Falsaperla M, Malaponte G et al (2005) Matrix metalloproteinases as diagnostic (MMP-13) and prognostic (MMP-2, MMP-9) markers of prostate cancer. Urol Res 33:44–50. https://doi.org/10.1007/s00240-004-0440-8
Chinni SR, Sivalogan S, Dong Z et al (2006) CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: The role of bone microenvironment-associated CXCL12. Prostate 66:32–48. https://doi.org/10.1002/pros.20318
Yang B, Tang F, Zhang B et al (2014) Matrix metalloproteinase-9 overexpression is closely related to poor prognosis in patients with colon cancer. World J Surg Oncol 12:1–6. https://doi.org/10.1186/1477-7819-12-24
Piotrowski-Daspit AS, Tien J, Nelson CM (2016) Interstitial fluid pressure regulates collective invasion in engineered human breast tumors via Snail, vimentin, and E-cadherin. Integr Biol 8:319–331. https://doi.org/10.1039/c5ib00282f
Chang L, Graham PH, Hao J et al (2013) Acquisition of epithelialmesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. https://doi.org/10.1038/cddis.2013.407
Edlind MP, Hsieh AC (2014) PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J Androl 16:378–386. https://doi.org/10.4103/1008-682X.122876
LoRusso PM (2016) Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol 34:3803–3815. https://doi.org/10.1200/JCO.2014.59.0018
Ibanez E, Agliano A, Prior C et al (2012) The quinoline imidoselenocarbamate EI201 blocks the AKT/mTOR pathway and targets cancer stem cells leading to a strong antitumor activity. Curr Med Chem 19:3031–3043. https://doi.org/10.2174/092986712800672076
Chen YS, Wu MJ, Huang CY et al (2011) CD133/Src axis mediates tumor initiating property and epithelial-mesenchymal transition of head and neck cancer. PLoS ONE 6:1–12. https://doi.org/10.1371/journal.pone.0028053
Sun Y, Kong W, Falk A et al (2009) C0D133 (Prominin) negative human neural stem cells are clonogenic and tripotent. PLoS ONE 4:16–18. https://doi.org/10.1371/journal.pone.0005498
Barrantes-Freer A, Renovanz M, Eich M et al (2015) CD133 expression is not synonymous to immunoreactivity for AC133 and fluctuates throughout the cell cycle in glioma stem-like cells. PLoS ONE 10:1–16. https://doi.org/10.1371/journal.pone.0130519
Feng HL, Liu YQ, Yang LJ et al (2010) Expression of CD133 correlates with differentiation of human colon cancer cells. Cancer Biol Ther 9:216–223. https://doi.org/10.4161/cbt.9.3.10664
Acknowledgements
This work was financially supported by grants from Tabriz University of Medical Sciences, Tabriz, Iran. The authors would like to thank them for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aghajani, M., Mokhtarzadeh, A., Aghebati-Maleki, L. et al. CD133 suppression increases the sensitivity of prostate cancer cells to paclitaxel. Mol Biol Rep 47, 3691–3703 (2020). https://doi.org/10.1007/s11033-020-05411-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05411-9