Abstract
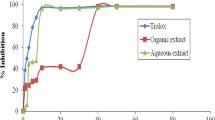
Different parts of Prunus persica as fruits, flowers, leaves and kernels have been consumed with dietary and therapeutic purposes traditionally. During fruit production, remarkable amount of leaves which can hold important bioactive groups as phenolics, have been left unutilized. The aim of this study was to investigate cytotoxic, antimicrobial and nitric oxide inhibitory activities of supercritical carbondioxide extracts of Prunus persica leaves. Among studied cell lines, supercritical carbon dioxide extract which was processed at 150 bar, 60 °C, and 6% co-solvent ethanol, exhibited remarkable cytotoxic activity against HeLa, MPanc-96 and MCF-7 cell lines with IC50 values of 12.22 µg/ml, 28.17 µg/ml and 35.51 µg/ml respectively, whereas IC50 value of conventional solvent extract was above 50 µg/ml. Minimum inhibitory concentration values determined for antibacterial and antifungal activities against Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Enterococcus faecium and Candida albicans were found as 62.50 µg/ml. Strong nitric oxide inhibition was achieved with IC50 of 9.30 µg/ml. The promising results revealed that Prunus persica leaves may have remarkable potential as supplement both for drug and food industries. This study is the first report revealing cytotoxic, antimicrobial and nitric oxide inhibitory activity of supercritical carbon dioxide extract of Prunus persica leaves.


Similar content being viewed by others
References
Gur I (2011) Seftali yetistiriciligi. Meyvecilik Arastirma Istasyonu Mudurlugu, Isparta
Gilani AH, Aziz N, Ali SM, Saeed M (2000) Pharmacological basis for the use of peach leaves in constipation. J Ethnopharmacol 73:87–93
The Food and Agriculture Organization. http://www.fao.org/faostat/. Accessed 4 Jan 2019
Shirosaki M, Koyama T, Yazawa K (2012) Suppressive effect of peach leaf extract on glucose absorption from the small intestine of mice. Biosci Biotechnol Biochem 76:89–94
Bhattacharjee C, Gupta D, Deb L, Kumar S, Debnath S, Dutta AS (2011) Effect of leave extract of Prunus persica Linn on acute inflammation in rats. Res J Pharmacogn Phytochem 3:38–40
Karadas O, Mese G, Ozcivici E (2018) Cytotoxic tolerance of healthy and cancerous bone cells to anti-microbial phenolic compounds depend on culture conditions. Appl Biochem Biotechnol 188:514–526
Yang H-H, Zhang C, Lai S-H, Zeng C-C, Liu Y-J, Wang X-Z (2017) Isoliquiritigenin ınduces cytotoxicity in PC-12 cells ın vitro. Appl Biochem Biotechnol 183:1173–1190
Song W, Qin S-T, Fang F-X, Gao Z-J, Liang D-D, Liu L-L, Tian H-T, Yang H-B (2018) Isolation and purification of condensed tannin from the leaves and branches of Prunus cerasifera and ıts structure and bioactivities. Appl Biochem Biotechnol 185:464–475
Arora DS, Mahajan H (2018) In vitro evaluation and statistical optimization of antimicrobial activity of Prunus cerasoides stem bark. Appl Biochem Biotechnol 184:821–837
Tarhan L, Nakipoğlu M, Kavakcıoğlu B, Tongul B, Nalbantsoy A (2016) The ınduction of growth ınhibition and apoptosis in HeLa and MCF-7 cells by Teucrium sandrasicum, having effective antioxidant properties. Appl Biochem Biotechnol 178:1028–1041
Roopan SM, Kumar SHS, Madhumitha G, Suthindhiran K (2015) Biogenic-production of SnO2 nanoparticles and ıts cytotoxic effect against hepatocellular carcinoma cell line (HepG2). Appl Biochem Biotechnol 175:1567–1575
Abrisqueta I, Conejero W, López-Martínez L, Vera J, Ruiz Sánchez MC (2017) Root and aerial growth in early-maturing peach trees under two crop load treatments. Span J Agric Res 15:18
Kazan A, Koyu H, Turu IC, Yesil-Celiktas O (2014) Supercritical fluid extraction of Prunus persica leaves and utilization possibilities as a source of phenolic compounds. J Supercrit Fluid 92:55–59
Yalcin HT, Ozen MO, Gocmen B, Nalbantsoy A (2014) Effect of Ottoman viper (Montivipera xanthina (Gray, 1849)) venom on various cancer cells and on microorganisms. Cytotechnology 66:87–94
Clinical and Laboratory Standards Institute (2009) Performance standards for antimicrobial susceptibility testing. In: Nineteenth informational supplement. Approved standard M100-S19. ed. Wayne, PA
Nalbantsoy A, Nesil T, Yılmaz-Dilsiz Ö, Aksu G, Khan S, Bedir E (2012) Evaluation of the immunomodulatory properties in mice and in vitro anti-inflammatory activity of cycloartane type saponins from Astragalus species. J Ethnopharmacol 139:574–581
Dhingra N, Kar A, Sharma R (2018) Inhibition of aromatase and cell proliferation of breast cancer and human placenta choriocarcinoma by Prunus persica extracts. Indian J Pharm Sci 80:903–910
Demir S, Turan I, Demir F, Ayazoglu Demir E, Aliyazicioglu Y (2017) Cytotoxic effect of Laurocerasus officinalis extract on human cancer cell lines. Mamar Pharm J 21:121–126
Meschini S, Pellegrini E, Condello M, Occhionero G, Delfine S, Condello G, Mastrodonato F (2017) Cytotoxic and apoptotic activities of Prunus spinosa trigno ecotype extract on human cancer cells. Molecules 22:1578
Manogna C, Bhaumik A, Haritha T, Nasreen S, Sucharitha M, Uttara M (2016) Evaluation of cytotoxic activity of various extracts of sweet cherry (Prunus avium) against human colorectal adenocarcinoma HT-29 cell line. Int J Chem Stud 4:17–21
Poongodi T, Srikanth R, Lalitha G (2015) Phytochemistry, GC-MS analysis and invitro cytotoxic activity of Prunus angustifolia leaves against MCF-7 breast cancer cell line. World J Pharm Pharm Sci 4:1489–1499
Jumaa AH, Hussein SM, Akafi L (2015) Study the in vitro effect of alcoholic extract of Prunus aremasia kernels, methotrexate, amygdalin and the combination between them on Hela cancer cell line. Iraqi J Cancer Med Gen 8:101–108
Maiyoa F, Moodleyb R, Singha M (2016) Phytochemistry, cytotoxicity and apoptosis studies of β-sitosterol-3-O-glucoside and β-amyrin from Prunus africana. Afr J Tradit Complement Altern Med 13:105–112
Vizzotto M, Porter W, Byrne D, Cisneros-Zevallos L (2014) Polyphenols of selected peach and plum genotypes reduce cell viability and inhibit proliferation of breast cancer cells while not affecting normal cells. Food Chem 164:363–370
Yu MH, Im HG, Lee SO, Sung C, Park DC, Lee IS (2007) Induction of apoptosis by immature fruits of Prunus salicina Lindl. cv. Soldam in MDA-MB-231 human breast cancer cells. Int J Food Sci Nutr 58:42–53
Fujii T, Ikami T, Xu J-W, Ikeda K (2006) Prune extract (Prunus domestica l.) suppresses the proliferation and induces the apoptosis of human colon carcinoma Caco-2. J Nutr Sci Vitam 52:389–391
Noratto G, Porter W, Byrne D, Cisneros-Zevallos L (2009) Identifying peach and plum polyphenols with chemopreventive potential against estrogen-independent breast cancer cells. J Agric Food Chem 57:5219–5226
Lea MA, Ibeh C, des Bordes C, Vizzotto M, Cisneros-Zevallos L, Byrne DH, Okie WR, Moyer MP (2008) Inhibition of growth and induction of differentiation of colon cancer cells by peach and plum phenolic compounds. Anticancer Res 28:2067–2076
Huang W-Y, Cai Y-Z, Zhang Y (2009) Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer 62:1–20
Ozpınar H, Dağ Ş, Yiğit E (2013) Şeftali (Persica vulgaris Miller) yaprak ekstraktının antibakteriyel etkisi. Cumhur Med J 35:172–178
Oyetayo AM, Bada SO (2017) Phytochemical screening and antibacterial activity of Prunus avium extracts against selected human pathogens. J Complement Altern Med 4:1–8
Arora DS, Mahajan H (2019) Major phytoconstituents of Prunus cerasoides responsible for antimicrobial and antibiofilm potential against some reference strains of pathogenic bacteria and clinical ısolates of MRSA. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-019-02985-4
Yaqeen Z, Naqvi NU, Sohail T, Rehman ZU, Fatima N, Imran H, Rehman A (2013) Screening of solvent dependent antibacterial activity of Prunus domestica. Pak J Pharm Sci 26:409–414
Rashid F, Ahmed R, Mahmood A, Ahmad Z, Bibi N, Kazmi SU (2007) Flavonoid glycosides from Prunus armeniaca and the antibacterial activity of a crude extract. Arch Pharm Res 30:932–937
Rovčanin BR, Ćebović T, Stešević D, Kekić D, Ristić M (2015) Antibacterial effect of Herniaria hirsuta, Prunus avium, Rubia tinctorum and Sempervivum tectorum plant extracts on multiple antibiotic resistant Escherichia coli. Biosci J 31:1852–1861
Yigit D, Yigit N, Mavi A (2009) Antioxidant and antimicrobial activities of bitter and sweet apricot (Prunus armeniaca L.) kernels. Braz J Med Biol Res 42:346–352
Singh P, Ahn S, Kang J-P, Veronika S, Huo Y, Singh H, Chokkaligam M, El-Agamy Farh M, Aceituno VC, Kim YJ, Yang D-C (2018) In vitro anti-inflammatory activity of spherical silver nanoparticles and monodisperse hexagonal gold nanoparticles by fruit extract of Prunus serrulata: a green synthetic approach. Artif Cells Nanomed Biotechnol 46:2022–2032
Sharma A, Joshi R, Kumar S, Sharma R, Rajneesh, Padwad Y, Gupta M (2018) Prunus cerasoides fruit extract ameliorates inflammatory stress by modulation of iNOS pathway and Th1/Th2 immune homeostasis in activated murine macrophages and lymphocytes. Inflammopharmacology 26:1483–1495
Tettey CO, Lincha VR, Lee DU, Yang IJ, Shin HM (2016) Anti-ınflammatory effects of the flowers of Prunus persica var. davidiana. J Food Biochem 40:227–234
Lee J, Yang G, Lee K, Lee MH, Eom JW, Ham I, Choi HY (2013) Anti-inflammatory effect of Prunus yedoensis through inhibition of nuclear factor-kappa B in macrophages. BMC Complement Altern Med 13:9
Rho JR, Jun CS, Ha YA, Yoo MJ, Cui MX, Baek HS, Lim JA, Lee YH, Chai KY (2007) Isolation and characterization of a new alkaloid from the seed of Prunus persica L. and its anti-inflammatory activity. Bull Korean Chem Soc 28:1289–1293
Kim SK, Kim HJ, Choi SE, Park KH, Choi HK, Lee MW (2008) Anti-oxidative and inhibitory activities on nitric oxide (NO) and prostaglandin E2 (COX-2) production of flavonoids from seeds of Prunus tomentosa Thunberg. Arch Pharm Res 31:424–428
Zhao X, Zhang W, Yin X, Su M, Sun C, Li X, Chen K (2015) Phenolic composition and antioxidant properties of different peach [Prunus persica (L.) Batsch] cultivars in China. Int J Mol Sci 16:5762
Saidani F, Giménez R, Aubert C, Chalot G, Betrán JA, Gogorcena Y (2017) Phenolic, sugar and acid profiles and the antioxidant composition in the peel and pulp of peach fruits. J Food Compos Anal 62:126–133
Mezzomo N, Mileo BR, Friedrich MT, Martinez J, Ferreira SR (2010) Supercritical fluid extraction of peach (Prunus persica) almond oil: process yield and extract composition. Bioresour Technol 101:5622–5632
Acknowledgements
Access to the facilities of Novel Fluidic Technologies Laboratory at Department of Bioengineering, Pharmaceutical Sciences Research Center (FABAL) and IKCU EFAL at Faculty of Pharmacy are highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koyu, H., Kazan, A., Nalbantsoy, A. et al. Cytotoxic, antimicrobial and nitric oxide inhibitory activities of supercritical carbon dioxide extracted Prunus persica leaves. Mol Biol Rep 47, 569–581 (2020). https://doi.org/10.1007/s11033-019-05163-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05163-1