Abstract
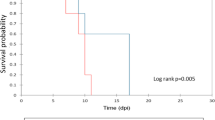
Different pig breeds have shown differential susceptibility to the pathogen infection; however, molecular mechanisms of the infection susceptibility are not fully understood. Streptococcus suis type 2 (SS2) is an important zoonotic pathogen. To identify the genes responsible for infection susceptibility, pigs from two different breeds (Enshi black and Landrace) were inoculated with SS2 and their spleen transcriptome profiles were investigated in the present study. The differentially expressed genes (DEGs) were analyzed from infected versus control pigs in each breed, and then compared between both pig breeds. Enshi black pig showed more DEGs than Landrace (830 vs. 611) and most of these were due to down-regulated genes (543 vs. 387). However some DEGs were uniquely expressed in one breed, some were expressed in opposite direction in both breeds. A number of candidate genes and pathways are identified which might be involved in susceptibility to SS2, for example, MMP9 and Resistin were only significantly expressed in Landrace. NPG3 and PMAP23 were up-regulated in Landrace whereas down-regulated in Enshi black. LENG8 in control Landrace have inherently higher expression than control Enshi black. IGKV6 is down-regulated in Landrace but up-regulated in Enshi black. Overall, the transcriptome profiles are consistent with the clinical signs, i.e. the Enshi black is more susceptible to SS2 infection than Landrace. This is the first study to identify differential gene expression between indigenous and modern commercial pigs after in vivo SS2 infection using RNA-seq. The significant DEGs in splenic profiles between two pig breeds suggested considerable involvement of genetic background in susceptibility to the SS2 infection in pigs.

Similar content being viewed by others
References
Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V (2012) The pig: a model for human infectious diseases. Trends Microbiol 20:50–57
Gottschalk M, Xu J, Calzas C, Segura M (2010) Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol 5:371–391
Fittipaldi N, Segura M, Grenier D, Gottschalk M (2012) Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol 7:259–279
Lian L, Qu LJ, Sun HY, Chen YM, Lamont SJ, Liu CJ, Yang N (2012) Gene expression analysis of host spleen responses to Marek’s disease virus infection at late tumor transformation phase. Poult Sci 91:2130–2138
Shin GW, White SL, Dahms HU, Jeong HD, Kim JH (2013) Disease resistance and immune-relevant gene expression in golden mandarin fish, Siniperca scherzeri Steindachner, infected with infectious spleen and kidney necrosis virus-like agent. J Fish Dis. doi:10.1111/jfd.12182
Li R, Zhang AD, Chen B, Liu T, Wang Y, Chen H, Jin M (2010) Response of swine spleen to Streptococcus suis infection revealed by transcription analysis. BMC Genom 11:556
Rong J, Zhang W, Wang X, Fan H, Lu C, Yao H (2012) Identification of candidate susceptibility and resistance genes of mice infected with Streptococcus suis type 2. PLoS One 7:e32150
Yu Y, Luo J, Mitra A, Chang S, Tian F, Zhang H, Yuan P, Zhou H, Song J (2011) Temporal transcriptome changes induced by MDV in Marek’s disease-resistant and -susceptible inbred chickens. BMC Genom 12:501
Smith J, Sadeyen JR, Paton IR, Hocking PM, Salmon N, Fife M, Nair V, Burt DW, Kaiser P (2011) Systems analysis of immune responses in Marek’s disease virus-infected chickens identifies a gene involved in susceptibility and highlights a possible novel pathogenicity mechanism. J Virol 85:11146–11158
Subramaniam S, Preeyanon L, Cheng HH (2013) Transcriptional profiling of Meq-dependent genes in Marek’s disease resistant and susceptible inbred chicken lines. PLoS One 8:e78171
Perumbakkam S, Muir WM, Black-Pyrkosz A, Okimoto R, Cheng HH (2013) Comparison and contrast of genes and biological pathways responding to Marek’s disease virus infection using allele-specific expression and differential expression in broiler and layer chickens. BMC Genom 14:64
Barreiro LB, Marioni JC, Blekhman R, Stephens M, Gilad Y (2010) Functional comparison of innate immune signalling pathways in primates. PLoS Genet 6:e1001249
Lunney JK, Chen H (2010) Genetic control of host resistance to porcine reproductive and respiratory syndrome virus (PRRSV) infection. Virus Res 154:161–169
Lunney JK, Steibel JP, Reecy JM, Fritz E, Rothschild MF, Kerrigan M, Trible B, Rowland RRR (2011) Probing genetic control of swine responses to PRRSV infection: current progress of the PRRS host genetics consortium. BMC Proc 5:S30
Reiner G, Willems H, Pesch S, Ohlinger VF (2010) Variation in resistance to the porcine reproductive and respiratory syndrome virus (PRRSV) in Pietrain and Miniature pigs. J Anim Breed Genet 127:100–106
Sousa KRS, Ribeiro AMF, Goes PRN, Guimarães SEF, Lopes PS, Veroneze R, Gasparino E (2011) Toll-Like Receptor 6 differential expression in two pig genetic groups vaccinated against Mycoplasma hyopneumoniae. BMC Proc 5:S9
Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25:1105–1111
Trapnell C, Williams BA, Pertea G (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515
Mortazavi A, Williams BA, McCue K (2008) Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat Methods 5:621–628
Audic S, Claverie JM (1997) The significance of digital gene expression profiles. Genome Res 10:986–995
Wang L, Feng Z, Wang X, Wang X, Zhang X (2010) DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26:136–138
Zhang Z (1986) Pig breeds in China. Shanghai Scientific and Technical Publishers, Shanghai
Li M, Tian S, Jin L et al (2013) Genomic analyses identify distinct patterns of selection in domesticated pigs and Tibetan wild boars. Nat Genet 45:1431–1438
Bierne H, Hamon M, Cossart P (2012) Epigenetics and Bacterial Infections. Cold Spring Harb Perspect Med 2:a010272
Gómez-Díaz E, Jordà M, Peinado MA, Rivero A (2012) Epigenetics of host–pathogen interactions: the road ahead and the road behind. PLoS Pathog 8:e1003007
Medzhitov R, Horng T (2009) Transcriptional control of the inflammatory response. Nat Rev Immunol 9:692–703
Bayarsaihan D (2011) Epigenetic mechanisms in inflammation. J Dent Res 90:9–17
Caldelari I, Chao Y, Romby P, Vogel J (2013) RNA-mediated regulation in pathogenic bacteria. Cold Spring Harb Perspect Med 3(9):a010298
Le Rhun A, Charpentier E (2012) Small RNAs in streptococci. RNA Biol 9:414–426
Chen H, Li C, Fang M, Zhu M, Li X, Zhou R, Li K, Zhao S (2009) Understanding Haemophilus parasuis infection in porcine spleen through a transcriptomics approach. BMC Genom 10:64
Huang Y, Huang X, Yan Y, Cai J, Ouyang Z, Cui H, Wang P, Qin Q (2011) Transcriptome analysis of orange-spotted grouper (Epinephelus coioides) spleen in response to Singapore grouper iridovirus. BMC Genom 12:556
Jobin MC, Gottschalk M, Grenier D (2006) Upregulation of prostaglandin E2 and matrix metalloproteinase 9 production by human macrophage-like cells: synergistic effect of capsular material and cell wall from Streptococcus suis. Microb Pathog 40:29–34
Parks WC, Wilson CL, Lopez-Boado YS (2004) Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 4:617–629
Page-McCaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8:221–233
Sbardella D, Fasciglione GF, Gioia M, Ciaccio C, Tundo GR, Marini S, Coletta M (2012) Human matrix metalloproteinases: an ubiquitarian class of enzymes involved in several pathological processes. Mol Aspects Med 33:119–208
Greenlee KJ, Corry DB, Engler DA, Matsunami RK, Tessier P, Cook RG, Werb Z, Kheradmand F (2006) Proteomic identification of in vivo substrates for matrix metalloproteinases 2 and 9 reveals a mechanism for resolution of inflammation. J Immunol 177:7312–7321
Chen H, Lunney JK, Cheng L, Li X, Cao J, Zhu M, Zhao S (2011) Porcine S100A8 and S100A9: molecular characterizations and crucial functions in response to Haemophilus parasuis infection. Dev Comp Immunol 35:490–500
Goyette J, Geczy CL (2010) Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids 41:821–842
Kadarmideen HN, Ali AA, Thomson PC, Muller B, Zinsstag J (2011) Polymorphisms of the SLC11A1 gene and resistance to bovine tuberculosis in African Zebu cattle. Anim Genet 42:656–658
Liu M, Fang L, Tan C, Long T, Chen H, Xiao S (2011) Understanding Streptococcus suis serotype 2 infection in pigs through a transcriptional approach. BMC Genom 12:253
Fabriek BO, Van Bruggen R, Deng DM, Ligtenberg AJ, Nazmi K, Schornagel K, Vloet RP, Dijkstra CD, van den Berg TK (2009) The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 113:887–892
Polfliet MM, Fabriek BO, Daniëls WP, Dijkstra CD, Van den Berg TK (2006) The rat macrophage scavenger receptor CD163: expression, regulation and role in inflammatory mediator production. Immunobiology 211:419–425
Dawson HD, Loveland JE, Pascal G et al (2013) Structural and functional annotation of the porcine immunome. BMC Genom 14:332
Milan G, Granzotto M, Scarda A, Calcagno A, Pagano C, Federspil G, Vettor R (2002) Resistin and adiponectin expression in visceral fat of obese rats: effect of weight loss. Obes Res 10:1095–1103
Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ (2005) Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun 334:1092–1101
Tang J, Wang C, Feng Y, Yang W et al (2006) Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med 3:e151
Schwartz JC, Lefranc MP, Murtaugh MP (2012) Evolution of the porcine (Sus scrofa domestica) immunoglobulin kappa locus through germline gene conversion. Immunogenetics 64:303–311
Butler JE, Wertz N, Sun XZ (2013) Antibody repertoire development in fetal and neonatal piglets. XIV. Highly restricted IGKV gene usage parallels the pattern seen with IGLV and IGHV. Mol Immunol 55:329–336
Schwartz JC, Lefranc MP, Murtaugh MP (2012) Organization, complexity and allelic diversity of the porcine (Sus scrofa domestica) immunoglobulin lambda locus. Immunogenetics 64:399–407
Wertz N, Vazquez J, Wells K, Sun J, Butler JE (2013) Antibody repertoire development in fetal and neonatal piglets. XII. Three IGLV genes comprise 70 % of the pre-immune repertoire and there is little junctional diversity. Mol Immunol 55:319–328
Sarson AJ, Parvizi P, Lepp D, Quinton M, Sharif S (2008) Transcriptional analysis of host responses to Marek’s disease virus infection in genetically resistant and susceptible chickens. Anim Genet 39:232–240
Acknowledgments
Thanks a lot to Dr. Nares Trakooljul (Leibniz Institute for Farm Animals Biology) in Germany for providing technical assistance to use the IPA software. This work was supported by National Key Project for Breeding of New Transgenic Varieties (2009ZX08009-141B) and Innovation system of agricultural science and technology in Hubei Province (2011-620-001-003) and open project from Hubei Key Lab for Animal Embryo Engineering and Molecular Breeding (2011ZD102).
Conflict of interest
The authors declare that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Additional information
U. Gaur, YY. Xiong and QP. Luo have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11033_2014_3680_MOESM2_ESM.doc
List of all DEGs: The list of all significant DEGs. There are 3 sheets: “Landrace” is DEGs in LI versus LC; “Enshi black” is DEGs in EI versus EC; “LC vs. EC” is DEGs in non-infected Landrace versus non-infected Enshi black. For each DEG is shown its Ensembl gene ID, fold-change, p value, adjusted q value (Benjamini-Hochberg correction), gene name and function description. Genes without names or descriptions are empty. (DOC 96 kb)
11033_2014_3680_MOESM4_ESM.tif
Canonical_Pathways: Significant canonical pathways based on DEGs identified by Fishers test using IPA. There are 3 sheets: “Landrace”, “Enshi black”, “LC vs. EC” which has the same meanings as S2. The ratio indicates the number of DEGs involved in each canonical pathway divided by the total number of genes/molecules within each pathway according to the IPA® Knowledge Base. (TIFF 1101 kb)
11033_2014_3680_MOESM5_ESM.tif
Significant GO_categories: GO categories identified according to p values using IPA. There are 3 sheets: the “Landrace”, “Enshi black”, “LC vs. EC” which has the same meanings as S2. (TIFF 19742 kb)
11033_2014_3680_MOESM7_ESM.tif
The top 20 up- & down-regulated DEGs in Landrace: The top 20 up- and down-regulated DEGs in Landrace piglet spleen following SS2 infection, meanwhile showed the expression status in Enshi black piglets. (TIFF 13820 kb)
11033_2014_3680_MOESM8_ESM.xls
The top 20 up- & down-regulated DEGs in Enshi: The top 20 up- and down-regulated DEGs in Enshi black piglet spleen following SS2 infection, meanwhile showed the expression status in Landrace piglets. (XLS 23 kb)
11033_2014_3680_MOESM9_ESM.xls
The top 20 up- & down-regulated DEGs in Landrace versus Enshi: The top 20 up DEGs in non-infected Landrace versus non-infected Enshi black piglet spleen. (XLS 370 kb)
11033_2014_3680_MOESM10_ESM.doc
The genes expressed in opposite directions in Landrace and Enshi black, Shows the DEG results of non-infected Landrace versus non-infected Enshi black. (DOC 31 kb)
11033_2014_3680_MOESM15_ESM.doc
Chart for gene ACTA1-related networks: This is merged Networks 4 and 23 in Landrace, and network 5 in Enshi black of S11. (DOC 86 kb)
Rights and permissions
About this article
Cite this article
Gaur, U., Xiong, Y., Luo, Q. et al. Breed-specific transcriptome response of spleen from six to eight week old piglet after infection with Streptococcus suis type 2. Mol Biol Rep 41, 7865–7873 (2014). https://doi.org/10.1007/s11033-014-3680-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3680-x