Abstract
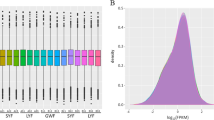
The ENO1 gene encodes a multifunctional enzyme that has been identified as a key component of the glycolytic pathway. Our previous studies demonstrated that ENO1 gene expression was higher in the ovaries of laying geese compared with prelaying geese. However, the molecular characterisation and expression profiling of the ENO1 gene in geese tissues and ovarian follicles remain to be determined. In this study, ENO1 cDNA (1,445 bp long) of the Sichuan white goose was cloned and characterised. The ORF of ENO1 cDNA is 1,305 bp in length and encodes a 434 amino acid protein with a molecular weight of 47.27 kDa. ENO1 expression in all of the examined tissues was the highest in spleen and the lowest in breast muscle. High expression of ENO1 appeared in the kidney, liver, adrenal gland, and retina. With increasing follicle growth, ENO1 gene expression began to decrease from the small white follicle to F5, which was followed by a sharp increase in expression in F4 and then a gradual decrease in expression from F3 to F1. Furthermore, in the postovulatory follicles (POF), the levels of ENO1 gene expression decreased gradually from POF1 to POF4. In conclusion, the ENO1 transcript was widely distributed in various tissues of the Sichuan white goose, but ENO1 expression was tissue-specific. Furthermore, the results of the ENO1 expression profiling of ovarian follicles suggest that ENO1 may play an important dual role in the progress of follicular development, where ENO1 acts as a glycolytic enzyme and also mediates apoptosis.







Similar content being viewed by others
References
Smolikova K, Mlynarcikova A, Scsukova S (2012) Role of interleukins in the regulation of ovarian functions. Endocr Regul 46(4):237–253
Ireland JJ, Zielak-Steciwko AE, Jimenez-Krassel F, Folger J, Bettegowda A, Scheetz D, Walsh S, Mossa F, Knight PG, Smith GW, Lonergan P, Evans AC (2009) Variation in the ovarian reserve is linked to alterations in intrafollicular estradiol production and ovarian biomarkers of follicular differentiation and oocyte quality in cattle. Biol Reprod 80(5):954–964
Shimizu T, Kaji A, Murayama C, Magata F, Shirasuna K, Wakamiya K, Okuda K, Miyamoto A (2012) Effects of interleukin-8 on estradiol and progesterone production by bovine granulosa cells from large follicles and progesterone production by luteinizing granulosa cells in culture. Cytokine 57(1):175–181
Harris SE, Adriaens I, Leese HJ, Gosden RG, Picton HM (2007) Carbohydrate metabolism by murine ovarian follicles and oocytes grown in vitro. Reproduction 134(3):415–424
Boland NI, Humpherson PG, Leese HJ, Gosden RG (1994) Characterization of follicular energy metabolism. Hum Reprod 9(4):604–609
Boland NI, Humpherson PG, Leese HJ, Gosden RG (1994) The effect of glucose metabolism on murine follicle development and steroidogenesis in vitro. Hum Reprod 9(4):617–623
Pancholi V (2001) Multifunctional alpha-enolase: its role in diseases. Cell Mol Life Sci 58(7):902–920
Piast M, Kustrzeba-Wojcicka I, Matusiewicz M, Banas T (2005) Molecular evolution of enolase. Acta Biochim Pol 52(2):507–513
Force A, Viallard JL, Saez F, Grizard G, Boucher D (2004) Electrophoretic characterization of the human sperm-specific enolase at different stages of maturation. J Androl 25(5):824–829
Nakamura N, Dai Q, Williams J, Goulding EH, Willis WD, Brown PR, Eddy EM (2013) Disruption of a spermatogenic cell-specific mouse enolase 4 (eno4) gene causes sperm structural defects and male infertility. Biol Reprod 88(4):90
Diaz-Ramos A, Roig-Borrellas A, Garcia-Melero A, Lopez-Alemany R (2012) Alpha-enolase, a multifunctional protein: its role on pathophysiological situations. J Biomed Biotechnol 2012:156795
Sugiura K, Eppig JJ (2005) Society for Reproductive Biology Founders’ Lecture 2005 Control of metabolic cooperativity between oocytes and their companion granulosa cells by mouse oocytes. Reprod Fertil Dev 17(7):667–674
Giallongo A, Oliva D, Cali L, Barba G, Barbieri G, Feo S (1990) Structure of the human gene for alpha-enolase. Eur J Biochem 190(3):567–573
Capello M, Ferri-Borgogno S, Cappello P, Novelli F (2011) Alpha-enolase: a promising therapeutic and diagnostic tumor target. FEBS J 278(7):1064–1074
Ghosh AK, Jacobs-Lorena M (2011) Surface-expressed enolases of Plasmodium and other pathogens. Mem Inst Oswaldo Cruz 106(Suppl 1):85–90
Kang B, Guo JR, Yang HM, Zhou RJ, Liu JX, Li SZ, Dong CY (2009) Differential expression profiling of ovarian genes in prelaying and laying geese. Poult Sci 88(9):1975–1983
Sugiura K, Pendola FL, Eppig JJ (2005) Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol 279(1):20–30
Gillott DJ, Eldib A, Iammarrone E, Leung KY, Thornhill AR, Grudzinskas JG (2008) Glycolytic enzyme expression in human granulosa cells. Fertil Steril 90(4 Suppl):1405–1410
Gerlt JA, Babbitt PC, Jacobson MP, Almo SC (2012) Divergent evolution in enolase superfamily: strategies for assigning functions. J Biol Chem 287(1):29–34
Song Z, Li Y, Liu Y, Xin J, Zou X, Sun W (2012) Alpha-enolase, an adhesion-related factor of Mycoplasma bovis. PLoS ONE 7(6):e38836
Lopez-Alemany R, Longstaff C, Hawley S, Mirshahi M, Fabregas P, Jardi M, Merton E, Miles LA, Felez J (2003) Inhibition of cell surface mediated plasminogen activation by a monoclonal antibody against alpha-Enolase. Am J Hematol 72(4):234–242
Redlitz A, Fowler BJ, Plow EF, Miles LA (1995) The role of an enolase-related molecule in plasminogen binding to cells. Eur J Biochem 227(1–2):407–415
Bae S, Kim H, Lee N, Won C, Kim HR, Hwang YI, Song YW, Kang JS, Lee WJ (2012) Alpha-enolase expressed on the surfaces of monocytes and macrophages induces robust synovial inflammation in rheumatoid arthritis. J Immunol 189(1):365–372
Aaronson RM, Graven KK, Tucci M, McDonald RJ, Farber HW (1995) Non-neuronal enolase is an endothelial hypoxic stress protein. J Biol Chem 270(46):27752–27757
Braithwaite T, Vugler A, Tufail A (2012) Autoimmune retinopathy. Ophthalmologica 228(3):131–142
Altenberg B, Greulich KO (2004) Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics 84(6):1014–1020
Hsiao KC, Shih NY, Fang HL, Huang TS, Kuo CC, Chu PY, Hung YM, Chou SW, Yang YY, Chang GC, Liu KJ (2013) Surface alpha-enolase promotes extracellular matrix degradation and tumor metastasis and represents a new therapeutic target. PLoS ONE 8(7):e69354
Chang GC, Liu KJ, Hsieh CL, Hu TS, Charoenfuprasert S, Liu HK, Luh KT, Hsu LH, Wu CW, Ting CC, Chen CY, Chen KC, Yang TY, Chou TY, Wang WH, Whang-Peng J, Shih NY (2006) Identification of alpha-enolase as an autoantigen in lung cancer: its overexpression is associated with clinical outcomes. Clin Cancer Res 12(19):5746–5754
Marcu KB, Bossone SA, Patel AJ (1992) Myc function and regulation. Annu Rev Biochem 61:809–860
Potter M, Marcu KB (1997) The c-myc story: where we’ve been, where we seem to be going. Curr Top Microbiol Immunol 224:1–17
Su YQ, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ (2008) Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 135(1):111–121
Leese HJ, Barton AM (1985) Production of pyruvate by isolated mouse cumulus cells. J Exp Zool 234(2):231–236
Onagbesan O, Bruggeman V, Decuypere E (2009) Intra-ovarian growth factors regulating ovarian function in avian species: a review. Anim Reprod Sci 111(2–4):121–140
Ucker DS, Jain MR, Pattabiraman G, Palasiewicz K, Birge RB, Li H (2012) Externalized glycolytic enzymes are novel, conserved, and early biomarkers of apoptosis. J Biol Chem 287(13):10325–10343
Jansen RP, de Boer K (1998) The bottleneck: mitochondrial imperatives in oogenesis and ovarian follicular fate. Mol Cell Endocrinol 145(1–2):81–88
Sutton-McDowall ML, Gilchrist RB, Thompson JG (2010) The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 139(4):685–695
Rieger D, Loskutoff NM (1994) Changes in the metabolism of glucose, pyruvate, glutamine and glycine during maturation of cattle oocytes in vitro. J Reprod Fertil 100(1):257–262
Johnson AL, Bridgham JT, Woods DC (2004) Cellular mechanisms and modulation of activin A- and transforming growth factor beta-mediated differentiation in cultured hen granulosa cells. Biol Reprod 71(6):1844–1851
Roberts R, Stark J, Iatropoulou A, Becker DL, Franks S, Hardy K (2004) Energy substrate metabolism of mouse cumulus-oocyte complexes: response to follicle-stimulating hormone is mediated by the phosphatidylinositol 3-kinase pathway and is associated with oocyte maturation. Biol Reprod 71(1):199–209
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (31201798) and by the Specialised Research Fund for the Doctoral Program of Higher Education (20105103120003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, B., Jiang, D.M., Bai, L. et al. Molecular characterisation and expression profiling of the ENO1 gene in the ovarian follicle of the Sichuan white goose. Mol Biol Rep 41, 1927–1935 (2014). https://doi.org/10.1007/s11033-014-3039-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3039-3