Abstract
Recent studies have demonstrated that vegetable rich diets have protective effects on the occurrence and prognosis of various cancers. In addition to dietary intakes, ascorbic acid and β-carotene are also taken as supplements. The aim of this study was to assess effects of ascorbic acid, β-carotene and their combinations on human hepatocellular carcinoma cell line HepG2. Ascorbic acid and β-carotene were applied to cells as plasma peak concentrations (70 and 8 μM, respectively) and their half concentrations (35 and 4 μM, respectively) for 24 and 48 h. Genotoxic and cytotoxic effects of ascorbic acid and β-carotene were evaluated by alkali single cell gel electrophoresis (SCGE), acridine orange/ethidium bromide staining patterns of cells (apoptosis and necrosis) and lipid peroxidation (thiobarbituric acid reactive substances, TBARS). Results of the SCGE demonstrated that both ascorbic acid and β-carotene caused DNA damage on HepG2 which were also concordant to increased apoptosis and necrosis of cells. Increased TBARS values also demonstrated increased lipid peroxidation in these cells. Results of the present study demonstrates that when dietary intakes of ascorbic acid and β-carotene and their relevant achievable plasma level concentrations were considered, both ascorbic acid and β-carotene induce genotoxic and cytotoxic damage on HepG2 together with increased oxidative damage in contrast to their protective effect on healthy cells. This may be correlated to oxidative status and balance of ROS in hepatocellular carcinoma cells.
Similar content being viewed by others
Introduction
Relation between consumption of vegetable rich diets and cancer occurrence and prognosis has been one of the important issues for many centuries. Interestingly, there are historical reports on discussions of “Cato the elder” (BC 234-149) on the issue. Today, epidemiological researches on cancer and diet have been supporting the historical previsions [1, 2].
In fact, dietary nutrients are combinations of pro- and antioxidants. Increase of reactive oxygen species (ROS) in cells may be cause of impaired mitochondrial electron transport, inflammatory reactions, cellular damage or exposure of cells to various toxic agents even including the air pollution [3]. Increase of ROS in healthy cells may affect various control mechanism of cells including cell cycle, DNA damage repair and apoptosis and may in turn cause tumor formation in different organs including gastrointestinal system [2, 3]. Cells have either enzymatic (e.g., superoxide dismutase, catalase, glutathione peroxidase) or non-enzymatic (e.g., vitamins, uric acid, glutathione) protective mechanisms against oxidative damage caused by ROS [3].
Herbal nutrients are composed of many bioactive compounds and in general, these compounds are believed to have effects on decreasing the risk of cancer prognosis [4]. Many people are recently using herbal based dietary nutrient supplements. Clinical studies of the last 30 years on the issue have proven protective effects of individual or combinational antioxidant and mineral supplements against cancer and other chronic diseases. On the contrary, some results of the previous studies demonstrated that vitamin and mineral supplements did not have any preventive role in development of primary tumors. Since development of malignant tumors may exceed clinical research period and heterogeneity of the subjects may cause unpredictable outcomes, individual or combination of antioxidant compounds should also be studied in model established cell lines especially for in the case of cancer [4, 5].
Ascorbic acid (AsA) and β-carotene (BC) are well defined for their antioxidant effect in cells against ROS production which effect cellular proliferation pathways and effect development and prognosis of carcinogenesis [4, 5]. AsA (vitamin C, ascorbate) is an essential dietary nutrient required as a co-factor for many enzymes and a very efficient antioxidant, scavenging reactive oxygen and nitrogen species and protecting cells against free radical-mediated damage. Besides exerting antioxidant influence directly, vitamin C can promote the removal of oxidative DNA damage from the DNA and/or nucleotide pool, through the upregulation of repair enzymes [6]. BC, which is the principal source of vitamin A for most of the world’s population, is effective in the neutralization of singlet oxygen and for the inhibition of oxidation by peroxide compounds [7].
Aim of the present study was to assess effects of in vitro applications of AsA, BC and their combinations on human hepatocellular carcinoma cell line HepG2 in terms of DNA damage, apoptosis/necrosis and oxidative damage as concentrations relevant to previously reported peak plasma levels of dietary intakes.
Materials and methods
Cell culture and treatments
In the present study, HepG2 human hepatocellular carcinoma cells, which are capable of metabolizing many nutrients, metabolites and xenobiotics, was used. In addition to secretion of plasma proteins and lipoproteins, these cells have also inductive P450 cytochromes [8]. Cells were maintained as an attached type monolayer culture in Dulbecco’s minimal essential medium (DMEM; Biochrom AG, Germany) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Biochrom AG), l-glutamine (Biochrom AG) and antibiotics (Biological Industries, Israel) at 37°C in a humidified atmosphere of a 5% CO2 (Heraeus, Hanau, Germany). Stock solutions of l-ascorbic acid (Sigma–Aldrich USA) and BC (Sigma–Aldrich USA) were prepared in distilled water and ethanol (EtOH), respectively. Control groups were assayed without any treatment in each experiment. Ethanol was also applied in separate flasks in each experiment (EtOH controls), in order to determine solvent effect on experimental setups. AsA and BC were applied to HepG2 cells as previously reported relevant achievable peak plasma level and half concentrations as 70–35 μM AsA, and 8–4 μM BC for 24 and 48 h [9, 10]. Correspondingly, 8–4 μM EtOH were applied to cells for 24 and 48 h.
Alkaline comet assay (alkaline single cell gel electrophoresis; SCGE)
For the determination of genotoxic effects AsA, BC and AsA-BC treatments on HepG2 cells alkaline SCGE was performed as previously described [8]. In brief, trypsinized cells were resuspended in 0.5 ml phosphate buffered saline (PBS) and 5 μl of cell suspension was mixed with 35 μl of 1% (w/v) low melting point agarose (LMPA; Sigma–Aldrich, USA) and added on to the slides coated with 0.5% (w/v) normal melting point agarose (NMPA; Sigma–Aldrich, USA). Cover slips were placed and slides were incubated on ice packs until the solidification of the agarose. Cover slips were removed and 40 μl 1% (w/v) LMPA was added on to the slides. Slides were incubated in lysis solution (2.5 M NaCl, 100 mM EDTA disodium salt, 10 mM Tris; pH 10) at 4°C (dark) for 2 h. Slides were incubated in electrophoresis buffer (300 mM NaOH, 1 mM EDTA disodium salt; pH > 13) for 20 min at dark and electrophoresis was performed at 24 V (300 mA) for 30 min. After neutralization (0.4 M Tris; pH 7.5), slides were stained with 2 μg/ml EtBr and observed under fluorescence microscope (Nicon, Eclips 600, Japan). A computerized image analysis system (Comet Assay IV; Perceptive Instruments, UK) was employed. Olive tail moment (TM) was used as the measure of DNA damage. A minimum of three SCGE slides were prepared for each treatment and in total, 100 nuclei were analyzed per treatment [8].
Assessment of apoptosis and necrosis by acridine orange/ethidium bromide staining
Apoptosis, necrosis and viable cells were differentiated after staining with a modified acridine orange/ethidium bromide (AO/EtBr) double staining [11]. Trypsinized cells were resuspended in 0.5 ml PBS and cell suspension was mixed with AO (4 μg/ml) and EtBr (0.5 μg/ml). The suspension was immediately examined by fluorescence microscopy (Nikon, Eclips 600, Japan) at 400× magnification. A minimum of 300 cells was counted for every treatment.
Lipid peroxidation
Lipid oxidation was determined by the modified method of Ahn et al. [12]. Cells were seeded to 25 cm2 culture flasks and treated with AsA, BC and AsA and BC for 24 and 48 h. Trypsinized cells were resuspended in 1 ml PBS and lysed with repetitive freezing and thawing at −20°C with vigorous vortexing in between freezing and thawing. Protein amount of lysates were determined according to Bradford method [13]. Twenty-five microliters of 0.04 M butylated hydroxytoluene (BHT; Sigma–Aldrich, USA) in ethanol was added to prevent artificial increase of malondialdehyde during the experiment. After addition of 0.5 ml 30% (w/v) trichloro acetic acid (TCA; Sigma–Aldrich, USA), suspension was vortexed. Three mililiters of thiobarbituric acid (TBA; Sigma–Aldrich, USA)/TCA (20 mM TBA in 15% (w/v) TCA) solution was added and the samples were vortex mixed. Samples were incubated in boiling water for 1 h for the development of color. Samples were cooled to room temperature on ice and centrifuged at 4000 rpm for 5 min. Absorbance of the supernatants were measured at 532 nm and TBA reactive substance (TBARS) concentrations were determined by using extinction coefficient as 155 mM−1 cm−1. All data of the lipid peroxidation were obtained from triplicate experiments and expressed as nmole TBARS per mg protein.
Statistical analysis
All data are expressed as mean ± standard error of the means (SE). All statistical analysis was performed using SPSS 11.5 Software (SPSS Inc., USA). Shapiro–Wilk test was used to test normality of distributions and homogeneity of variances was tested by Levene’s test. In case of normal distribution and homogeneity of variances, treatments were statistically evaluated by one-way ANOVA analysis at 0.05 levels and post hoc Tukey analysis were carried out to find groups whose mean differences were significant. Differences between 24 and 48 h in treatments were statistically evaluated by independent-samples t-test at the 0.05 level. If data were not normally distributed or variances were not homogenous, Mann–Whitney U or Kruskal–Wallis with post hoc Dunn’s test was used at 0.05 levels for the comparison of incubation periods and treatments, respectively.
Results
Genotoxic effects of AsA and BC on HepG2 cells
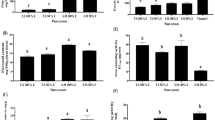
After 24 and 48 h of treatment with AsA and β-carotene, the comet assay was conducted (Fig. 1). With relevant plasma level and half plasma level concentrations of AsA (70 and 35 μM, respectively), the mean tail moment in HepG2 nuclei significantly increased from 1.73 ± 0.23 μm (control) to 7.53 ± 0.68 and 5.83 ± 0.43 μm after 24 h treatment, respectively. In addition, 24 h of 70 μm AsA treatment caused significantly higher DNA damage when compared to 35 μm AsA treatments (Fig. 1b). When treatment duration was increased to 48 h, though the DNA damage induced (4.37 ± 0.74 and 2.83 ± 0.50 μm for 70 and 35 μm, respectively) was significantly higher than the control (1.88 ± 0.08 μm), AsA induced DNA damage significantly decreased (with respect to 24 h treatment groups. When AsA was applied in combination with BC for 24 h, there was not any significant change in mean tail moments in comparison to 70 μm AsA treatment where the levels significantly higher than that of 35 μm AsA treatment group. AsA induced DNA damage did not change when same AsA concentrations were applied with BC for 48 h; however the levels varied between different concentration treatment groups (Fig. 1b). In contrast to AsA, BC application in plasma level relevant concentrations induced concentration dependent but incubation period independent DNA damage in nuclei of HepG2 cells (Fig. 1c). Since BC stock solutions were prepared in ethanol, solvent effect of ethanol was evaluated for DNA damage. After 24 h of EtOH application there was not any significant change in the mean tail moments of EtOH applications (1.43 ± 0.15 and 1.82 ± 0.3 μm for EtOH and EtOH/2, respectively) when compared to control. Similarly, the values were 1.64 ± 0.25 and 1.71 ± 0.18 μm for EtOH and EtOH/2, respectively, which were statistically insignificant when compared to 48 h control groups. BC treatment induced DNA damages were significantly higher in comparison to combination applications of AsA and BC for both 24 and 48 h of treatments. In addition, BC induced considerably higher tail moment when compared to AsA in both 24 and 48 h of applications (except for the 24 h AsA/2 and BC/2 treatment groups).
a Representative micrographs for comet evaluation; and induction of DNA damage in treated HepG2 cells. b Effect of AsA and AsA + BC on tail moments; *donations represent significant difference between 24 h treatment groups (i.e., *control versus other treatments, **AsA versus AsA/2, ***AsA/2 versus AsA + BC, (AsA + BC)/2) and ×donations represent significant difference between 48 h treatment groups (i.e., ×control versus other treatments, ××AsA versus AsA/2, (AsA + BC)/2, ×××AsA/2 versus AsA + BC, ××××AsA + BC versus AsA + BC)/2); c Effect of BC and AsA + BC on tail moments; *donations represent significant difference between 24 h treatment groups (i.e., *control versus other treatments, **BC versus BC/2, AsA + BC, (AsA + BC)/2, ***BC/2 versus AsA + BC, (AsA + BC)/2), and ×donations represent significant difference between 48 h treatment groups (i.e., ×control versus other treatments, ××BC versus BC/2, AsA + BC, (AsA + BC)/2, ×××BC/2 versus (AsA + BC)/2, ××××AsA + BC versus AsA + BC)/2). Letters represent significant difference in between 24 and 48 h incubation periods
AsA and BC induced apoptosis and necrosis of HepG2 cells
When results of the acridine orange/ethidium bromide staining were evaluated (Table 1), single and combination applications of AsA and BC induced apoptosis in HepG2 cells in 24 and 48 h when compared to untreated cells. 70 μM AsA induced apoptosis and necrosis were significantly higher of than that of 35 μM for both 24 and 48 h treatments. Likewise, 8 μM BC induced apoptosis and necrosis were significantly higher of than that of 4 μM for both 24 and 48 h treatments. When both AsA and BC were applied to cells, apoptosis were not different for 24 and 48 h whereas after 48 h statistically significant increased apoptosis was observed when 35 μM AsA and 4 μM BC were applied in combination. On the contrary, when two agents were used together necrotic cell rates decreased in both time and dose dependent manner. Furthermore, combined applications of agents caused neither increased apoptosis nor necrosis in comparison to single applications of AsA and BC. When EtOH treatment groups were evaluated after 24 h, results obtained (apoptotic cells were 3.59 ± 0.6 and 2.87 ± 0.23% for EtOH and EtOH/2, respectively; and necrotic cells were 4.12 ± 0.56 and 4.31 ± 0.48% for EtOH and EtOH/2, respectively) were not statistically different from control groups (Table 1). The results were similar for 48 h treatments, i.e. apoptotic cells were 3.17 ± 0.62 and 3.87 ± 0.42% for EtOH and EtOH/2, respectively; and necrotic cells were 4.91 ± 0.41 and 4.411 ± 0.51% for EtOH and EtOH/2, respectively.
Lipid peroxidation levels in treated HepG2 cells
With relevant plasma level and half plasma level concentrations of AsA (70 and 35 μM, respectively), the TBARS levels in HepG2 significantly increased from 0.33 ± 0.01 nmol/mg protein (control) to 1.30 ± 0.05 and 0.75 ± 0.07 nmol/mg protein after 24 h treatment, respectively. In addition, 24 h of 70 μm AsA treatment caused significantly higher lipid peroxidation when compared to 35 μm AsA treatments (Fig. 2a). When treatment duration was increased to 48 h, though the TBARS levels (0.95 ± 0.01 and 0.052 ± 0.04 nmol/mg protein for 70 and 35 μm, respectively) were significantly higher than the control (0.34 ± 0.01 nmol/mg protein), AsA induced oxidative damage significantly decreased with respect to 24 h treatment groups. In addition, decreasing AsA concentration caused significant decrease of lipid peroxidation for both 24 and 48 h treatments. When 70 μM AsA was applied in combination 8 μM with BC for 24 h, there was not any significant change in TBARS levels in comparison to 70 μm AsA treatments where the levels were significantly higher than that of half concentration values of combination treatment. Similar to AsA treatments, combination treatments of AsA and BC caused concentration dependent increase in TBARS values (0.83 ± 0.11 and 0.46 ± 0.09 nmol/mg protein for AsA + BC and (AsA + BC)/2, respectively). Furthermore, similar to 70 μm AsA treatment, 70 μm AsA and 8 μm BC treatment caused significantly higher levels of lipid peroxidation in 24 h when compared to 48 h. According to Fig. 2b, 8 and 4 μm BC applications increased TBARS levels in concentration dependent but incubation period independent manner (i.e., 1.49 ± 0.06 and 0.71 ± 0.03 nmol/mg protein for 48 h, and 1.49 ± 0.03 and 0.81 ± 0.01 nmol/mg protein for 48 h). Solvent effect of ethanol was insignificant (i.e. 0.41 ± 0.09 and 0.38 ± 0.03 nmol/mg protein for 24 h EtOH and EtOH/2, respectively; and 0.29 ± 0.05 and 0.36 ± 0.04 nmol/mg protein for 48 h EtOH and EtOH/2, respectively). Eight μm BC treatment caused significantly higher lipid peroxidation than combination applications of AsA and BC in 48 h where the levels were significantly higher than 35 μm AsA and 4 μm BC but indifferent from 70 μm AsA and 8 μm BC in 24 h. Conversely, when BC was lowered to 4 μm, lipid peroxidation was indifferent from 35 μm AsA and 4 μm BC but significantly lower than that of 70 μm AsA and 8 μm BC in 24 h where the levels were statistically indifferent in 48 h treatment groups.
Lipid peroxidation (TBARS) levels a *donations represent significant difference between 24 h treatment groups (i.e., *control versus other treatments, **AsA versus AsA/2, (AsA + BC)/2, ***AsA/2 versus AsA + BC, ****AsA + BC versus (AsA + BC)/2) and ×donations represent significant difference between 48 h treatment groups (i.e., ×control versus other treatments, ××AsA versus AsA/2, (AsA + BC)/2, ×××AsA + BC versus (AsA + BC)/2); b *donations represent significant difference between 24 h treatment groups (i.e., *control versus other treatments, **BC versus BC/2, AsA + BC, (AsA + BC)/2, ***BC/2 versus AsA + BC, ****AsA + BC versus (AsA + BC)/2); ×donations represent significant difference between 48 h treatment groups (i.e., ×control versus other treatments, ××BC versus BC/2, AsA + BC, (AsA + BC)/2, ×××AsA + BC versus (AsA + BC)/2). Letters represent significant difference in between 24 and 48 h incubation periods
Discussion
Ascorbic acid (vitamin C) is one of the most important hydrophilic ROS scavengers in extracellular fluid and protects biological membranes from peroxidation and oxidative damage by scavenging free radicals in liquid phase [14]. Likewise, carotenoids including β-carotene free radical scavenger antioxidants. In addition, they affect cellular processes such as cell proliferation, differentiation and receptor mediated cell signaling and malignant transformation, which is related to their chemo preventive effect as well [15].
Many in vitro studies demonstrated antioxidant effect of ascorbic acid and β-carotene in the presence of pro-oxidants, DNA damaging agents and mutagens which lead to malignant transformation [2, 5, 14, 16, 17]. On the other hand, there are some meta-analysis reports on lack of anticarcinogenic effect of these agents on gastrointestinal cancer incidence and mortality [3].
In this study, effects ascorbic acid and β-carotene were demonstrated in hepatocellular carcinoma cell line HepG2 in the absence of any other pro-oxidant compound. Beta-carotene concentration was determined considering dietary supplement daily intake of β-carotene and its peak plasma level concentrations as previously reported [9, 10].
Previous reports on fibroblasts demonstrated that ascorbic acid might prevent cellular proliferation and induce necrosis via targeting genes related to S phase of the cell cycle [18]. Prasad et al. [19] also reported that ascorbic acid had prevented proliferation of various human and mouse carcinoma cells dependent on concentration. Furthermore, it was shown to inhibit COX-2, p38 and IGF pathways and induce apoptosis. In melanoma cells [20], which are concordant to other findings on inhibitory effect of ascorbic acid on cell cycle. According to our results, ascorbic acid induced DNA damage, apoptosis and necrosis together with lipid peroxidation in dose dependent manner. However, in contrast to decreased DNA damage and necrosis, apoptosis increased when incubation period prolonged to 48 h (Fig. 1; Table 1).
Antioxidant capacity of β-carotene and ascorbic acid is dependent on oxygen pressure, their concentration, and combinational effects when applied together. Moreover, previous studies demonstrated pro-oxidant effect of β-carotene under effect of these factors [1, 3]. For example, 1–3 μM β-carotene was shown to protect HT-29 human colon carcinoma cells against DNA and membrane damage where 4–10 μM of application concentration its protective role was shown be diminished. Furthermore, in vitro cell culture conditions of 5% CO2 was also shown to increase pro-oxidant activity of β-carotene [21]. Results present in this study are also in concordance with these previous findings. Here, we demonstrate that β-carotene induced genotoxic and cytotoxic damage in HepG2 cells in dose and time dependent manner in the absence of any other oxidative damage inducing agents (Figs. 1, 2; Table 1).
AsA is a potent water-soluble antioxidant and can reduce most biologically relevant radicals and oxidants such as hydroxyl radical, superoxide anion, hypochlorous acid or singlet oxygen [22]. Similarly, BC deactivates reactive chemical species, such as singlet oxygen and free radicals that lead to lipid peroxidation [23]. Since AsA and BC have similar scavenging effects on same ROS, they seem to act through similar pathways in cells. In agreement with this, in the current study, the results obtained with the combined applications of AsA and BC were not higher from that of separate treatment of these antioxidants (Figs. 1, 2; Table 1).
Vitamin C has been reported to increase the efficacy of several chemotherapeutic drugs either in vitro or in vivo [22, 24]. Though, beneficial and chemo preventive effects of ascorbic acid supplementation seems to be supported by many clinical and in vitro studies two points have been considered on the issue; first one is about insufficiency of plasma ascorbate levels to kill tumor cells achieved with oral vitamin C supplementation [25], which may be overcome by intravenous supplementation at higher doses [26–28]. Secondly, higher concentration of ascorbate was recorded to accumulate in tumors than in normal tissues [29, 30]. This has raised the discussions as other preventive supplements that it can also protect tumor cells from oxidative damage and decrease efficiency of chemotherapy associated with increased ROS in tumor cells. However, Casciari et al. [31] demonstrated antitumor effect of ascorbic acid alone on cells in the SW620 hollow fiber in vitro solid tumor model at clinically achievable concentrations or in combination with doxorubicin. They have reported that the effect of ascorbate on doxorubicin efficacy was concentration dependent i.e., low doses were protective while high doses increased cell killing. There are in vivo researches on antitumor potency of β-carotene as well [reviewed in 32]. For example, Seifter et al. [33] reported that slight regression of transplanted adenocarcinoma of breast in rat after supplementation with high doses of β-carotene. Similarly, growth inhibition of oral carcinoma in hamsters has been reported when β-carotene was directly administrated adjacent to the tumor site [34, 35].
Here we demonstrate in vitro potential genotoxic and cytotoxic antitumor effect of ascorbic acid and β-carotene on HepG2 human hepatocellular carcinoma cells at achievable plasma level concentrations. Tough, more studies at molecular level and probably with other hepatocellular carcinoma cell lines are required this study gives a preliminary insight to further in vivo research, and first to our knowledge, on their potential effect on hepatocellular carcinoma.
References
Woods JA, Bilton RF, Young AJ (1999) Beta carotene enhances hydrogen peroxide-induced DNA damage in human hepatocellular HepG2 cells. FEBS Lett 23:255–258
Bjelakovic G, Nikolova D, Simonetti RG, Gluud C (2004) Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 2:1219–1228
Zhang P, Omaye ST (2001) DNA strand breakage and oxygen tension: effects of β-carotene, alpha-tocopherol and AsA. Food Chem Toxicol 39:239–246
Neuhouser ML (2004) Dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer 50:1–7
Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M et al (2009) Vitamins C and E and β carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst 7:14–23
Arranz N, Haza AI, García A, Rafter J, Morales P (2007) Protective effect of vitamin C towards N-nitrosamine-induced DNA damage in the single-cell gel electrophoresis (SCGE)/HepG2 assay. Toxicol In Vitro 21:1311–1317
Dias CD, Araújo BC, Dutra ES, Nepomuceno JC (2009) Protective effects of beta carotene against the genotoxicity of doxorubicin in somatic cells of Drosophila melanogaster. Genet Mol Res 17:1367–1375
O’Brien NM, Woods JA, Aherne SA, O’Callaghan YC (2000) Cytotoxicity, genotoxicity and oxidative reactions in cell-culture models: modulatory effects of phytochemicals. Biochem Soc Trans 28:22–26
VanderJagt DJ, Garry PJ, Bhagavan HN (1987) Ascorbic acid intake and plasma levels in healthy elderly people. Am J Clin Nutr 46:290–294
Micozzi MS, Brown ED, Edwards BK, Bieri JG, Taylor PR, Khachik F et al (1992) Plasma carotenoid response to chronic intake of selected foods and BC supplements in men. Am J Clin Nutr 55:1120–1125
Baskić D, Popović S, Ristić P, Arsenijević NN (2006) Analysis of cycloheximide-induced apoptosis in human leukocytes: fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol Int 30:924–932
Ahn DU, Olson DG, Jo C, Chen X, Cand Wu, Lee JI (1998) Effect of muscle type, packaging, and irradiation on lipid oxidation, volatile production, and color in raw pork patties. Meat Sci 49:27–39
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein–dye binding. Anal Biochem 72:248–254
García A, Morales P, Arranz N, Delgado ME, Rafter J, Haza AI (2009) Antiapoptotic effects of dietary antioxidants towards N-nitrosopiperidine and N-nitrosodibutylamine-induced apoptosis in HL-60 and HepG2 cells. J Appl Toxicol 29:403–413
Prakash P, Russell RM, Krinsky NI (2001) In vitro inhibition of proliferation of estrogen-dependent and estrogen-independent human breast cancer cells treated with carotenoids or retinoids. J Nutr 131:1574–1580
Li JJ, Tang Q, Li Y, Hu BR, Ming ZY, Fu Q et al (2006) Role of oxidative stress in the apoptosis of hepatocellular carcinoma induced by combination of arsenic trioxide and ascorbic acid. Acta Pharmacol Sin 27:1078–1084
van Helden YG, Keijer J, Heil SG, Picó C, Palou A, Oliver P et al (2009) BC affects oxidative stress-related DNA damage in lung epithelial cells and in ferret lung. Carcinogenesis 30:2070–2076
Belin S, Kaya F, Duisit G, Giacometti S, Ciccolini J, Fontés M (2009) Antiproliferative effect of Ascorbic acid is associated with the inhibition of genes necessary to cell cycle progression. PLoS One 4:e4409
Prasad KN, Kumar A, Kochupillai V, Cole WC (1999) High doses of multiple antioxidant vitamins: essential ingredients in improving the efficacy of standard cancer therapy. J Am Coll Nutr 18:13–25
Lee SK, Kang JS, Da Jung J, Hur DY, Kim JE, Hahm E et al (2008) Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J Cell Physiol 216:180–188
Lowe GM, Booth LA, Young AJ, Bilton RF (1999) Lycopene and Beta Carotene protect against oxidative damage in HT29 cells at low concentrations but rapidly lose this capacity at higher doses. Free Radic Res 30:141–151
Verrax J, Calderon PB (2008) The controversial place of vitamin C in cancer treatment. Biochem Pharmacol 15:1644–1652
Burton GW, Ingold KU (1984) Beta-carotene: an unusual type of lipid antioxidant. Science 11:569–573
Du J, Martin SM, Levine M, Wagner BA, Buettner GR, Wang SH, Taghiyev AF, Du C, Knudson CM, Cullen JJ (2010) Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin Cancer Res 16(2):509–520
Riordan NH, Riordan HD, Meng X, Li Y, Jackson JA (1995) Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med Hypotheses 44:207–213
Riordan HR, Jackson JA, Schultz M (1990) Case study: high-dose intravenous vitamin C in the treatment of a patient with adenocarcinoma of the kidney. J Ortho Med 5:5–7
Riordan HD, Jackson JA, Riordan NH, Schultz M (1998) High-dose intravenous vitamin C in the treatment of a patient with renal cell carcinoma of the kidney. J Ortho Med 13:72–73
Jackson JA, Riordan HD, Hunninghauke RE, Riordan N (1995) High dose intravenous vitamin C and long time survival of a patient with cancer of the head of the pancreas. J Ortho Med 10:87–88
Langemann H, Torhorst J, Kabiersch A, Krenger W, Honegger CG (1989) Quantitative determination of water- and lipid-soluble antioxidants in neoplastic and non-neoplastic human breast tissue. Int J Cancer 15:1169–1173
Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M (2005) Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA 102(38):13604–13609
Casciari JJ, Riordan NH, Schmidt TL, Meng XL, Jackson JA, Riordan HD (2001) Cytotoxicity of ascorbate, lipoic acid, and other antioxidants in hollow fibre in vitro tumours. Br J Cancer 1:1544–1550
Nishinoa H, Murakoshic M, Tokudad H, Satomid Y (2009) Cancer prevention by carotenoids. Arch Biochem Biophys 483(2):165–168
Seifter E, Rettura A, Padawar J, Levenson SM (1984) Vitamin A and β-carotene as adjunctive therapy to tumor excision, radiation therapy and chemotherapy. In: Prasad KN (ed) Vitamins, nutrition and cancer. Karger, Basel, pp 1–19
Schwartz JL (1995) Molecular and biochemical control of tumor growth following treatment with carotenoids or tocopherols. In: Prasad KN, Santamaria L, Williams RM (eds) Nutrients in cancer prevention and treatment. Humana Press, Totawa, NJ, pp 287–316
Shklar G (1995) Inhibition and regression of experimental oral cancer by beta carotene and vitamin E: emerging concepts. In: Prasad KN, Santamaria L, Williams RM (eds) Nutrients in cancer prevention and treatment. Humana Press, Totowa, NJ, pp 317–333
Acknowledgments
This study was approved by Baskent University Institutional Review Board (Project No. DA10/01) and supported by Baskent University Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yurtcu, E., Iseri, O.D. & Sahin, F.I. Effects of ascorbic acid and β-carotene on HepG2 human hepatocellular carcinoma cell line. Mol Biol Rep 38, 4265–4272 (2011). https://doi.org/10.1007/s11033-010-0549-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0549-5