Abstract
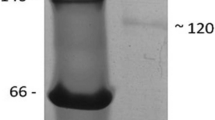
The lipopolysaccharide and β-1,3-glucan-binding protein (LGBP) plays an important function in the innate immune response of invertebrates as a pattern recognition receptor (PRR). Herein, we described the isolation and characterization of pearl oyster Pinctada fucata LGBP (designated as poLGBP). The poLGBP cDNA was 2,075 bp long and consisted of a 5′-untranslated region (UTR) of 18 bp, a 3′-UTR of 299 bp with one cytokine RNA instability motifs (ATTTA), and an open reading frame (ORF) of 1,758 bp encoding a polypeptide of 585 amino acids with an estimated molecular mass of 65.1 kDa and a theoretical isoelectric point of 5.80. Homology analysis of the deduced amino acid sequence of the poLGBP with other known LGBP sequences by MatGAT software revealed that the poLGBP shared 26.3–56.7% identity and 40.5–70.9% similarity to the other known LGBP sequences. SMART and alignment analysis revealed that the poLGBP possessed a potential polysaccharide-binding motif, a glucanase motif, a LPS-binding site, a β-1,3-linkage of polysaccharide, a glycine-rich region, a threonine-rich region and two N-glycosylation sites. In healthy pearl oyster, the poLGBP mRNA was specifically expressed in digestive gland, and not detected in gill, adductor muscle, gonad, intestine, mantle and hemocytes. However, after bacteria stimulation, the expression of the poLGBP mRNA was significantly up-regulated in digestive gland and also weakly detected in haemocytes, gonad and intestine. After LPS stimulation, the poLGBP mRNA expression was significantly up-regulated at 8 and 12 h in digestive gland, and the expression level was 10.7-fold higher than the PBS group at 12 h. After bacteria stimulation, the expression level of the poLGBP mRNA was also significantly up-regulated in digestive gland and was 12.9-fold higher than the PBS group at 8 h. However, during the experiment, the poLGBP mRNA expression was not detected in gill after LPS or bacteria stimulation. The tissue-specific expression and the expression up-regulation after LPS or bacteria stimulation in digestive gland suggested that the poLGBP was an inducible acute-phase protein and might play an important function in digestion as digestive enzyme and pattern recognition receptor.




Similar content being viewed by others
References
Wu X, Pan J (1999) Studies on rickettsia-like organism disease of the tropical marine pearl oyster I: the fine structure and morphogenesis of pinctada maxima pathogen rickettsia-like organism. J Invertebr Pathol 73(2):162–172
Lau KW, Ren J, Wai NL, Lau SC, Qian PY, Wong PK, Wu M (2006) Marinomonas ostreistagni sp. nov., isolated from a pearl-oyster culture pond in Sanya, Hainan Province, China. Int J Syst Evol Microbiol 56(10):2271–2275
Wang AM, Shi YH, Wu X (2004) The comparison among effects of four treatments to reduce polychaete infestation in pearl oyster, Pinctada martensi. Mar Fish Res 25(2):41–46
Iwanaga S, Lee BL (2005) Recent advances in the innate immunity of invertebrate animals. J Biochem Mol Biol 38(2):128–150
Medzhitov R, Janeway CA Jr (1997) Innate immunity: the virtues of a nonclonal system of recognition. Cell 91:295–298
Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20:197–216
Muta T, Miyata T, Misumi Y, Tokunaga F, Nakamura T, Toh Y, Ikehara Y, Iwanaga S (1991) Limulus factor C: an endotoxin-sensitive serine protease zymogen with a mosaic structure of complement-like, epidermal growth factor-like, and lectin-like domains. J Biol Chem 266(10):6554–6561
Seki N, Muta T, Oda T, Iwaki D, Kuma K, Miyata T, Iwanaga S (1994) Horseshoe crab (1,3)-h-d-glucan sensitive coagulation factor G. A serine protease zymogen heterodimer with similarities to β-glucan-binding proteins. J Biol Chem 269(2):1370–1374
Cerenius L, Liang Z, Duvic B, Keyser P, Hellman U, El Palva T, Iwanaga S, Soderhall K (1994) Structure and biological activity of a 1,3-beta-d-glucan-binding protein in crustacean blood. J Biol Chem 269(47):29462–29467
Jomori T, Natori S (1992) Function of the lipopolysaccharide-binding protein of Periplaneta americana as an opsonin. FEBS Lett 296(3):283–286
Johansson MW (1999) Cell adhesion molecules in invertebrate immunity. Dev Comp Immunol 23(4–5):303–315
Liu F, Li F, Dong B, Wang X, Xiang J (2009) Molecular cloning and characterization of a pattern recognition receptor, lipopolysaccharide and beta-1,3-glucan binding protein (LGBP) from Chinese shrimp Fenneropenaeus chinensis. Mol Biol Rep 36(3):471–477
Du XJ, Zhao XF, Wang JX (2007) Molecular cloning and characterization of a lipopolysaccharide and β-1,3-glucan binding protein from fleshy prawn (Fenneropenaeus chinensis). Mol Immunol 44:1085–1094
Lin YC, Vaseeharan B, Chen JC (2008) Identification and phylogenetic analysis on lipopolysaccharide and β-1,3-glucan binding protein (LGBP) of kuruma shrimp Marsupenaeus japonicus. Dev Comp Immunol 32(11):1260–1269
Cheng W, Liu CH, Tsai CH, Chen JC (2005) Molecular cloning and characterization of a pattern recognition molecule, lipopolysaccharide- and beta-1,3-glucan binding protein (LGBP) from the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 18(4):297–310
Yu XQ, Zhu YF, Ma C, Fabrick JA, Kanost MR (2002) Pattern recognition receptors in Manduca sexta plasma. Insect Biochem Mol Biol 32(10):1287–1293
Bilei M, De Baetselier P, Van Dijck E, Stijlemans B, Colige A, Beschin A (2001) Distinct carbohydrate recognition domains of an invertebrate defense molecule recognize Gram-negative and Gram-positive bacteria. J Biol Chem 276(49):45840–45847
Lee SY, Wang R, Söderhäll K (2000) A lipopolysaccharide- and beta-1,3-glucan-binding protein from hemocytes of the freshwater crayfish Pacifastacus leniusculus. Purification, characterization, and cDNA cloning. J Biol Chem 275(2):1337–1343
Su JG, Song LS, Xu W, Wu LT, Li HL, Xiang JH (2004) cDNA cloning and mRNA expression of the lipopolysaccharide- and beta-1,3-glucan-binding protein gene from scallop Chlamys farreri. Aquaculture 239:69–80
Nikapitiya C, De Zoysa M, Lee J (2008) Molecular characterization and gene expression analysis of a pattern recognition receptor from disk abalone, Haliotis discus discus. Fish Shellfish Immunol 25:638–647
Campanella JJ, Bitincka L, Smalley J (2003) MatGat: an application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinformatics 4(29):1–4
Schultz J, Milpetz F, Bork P, Ponting CP (1998) SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA 95:5857–5864
Letunic I, Copley RP, Pils B, Pinkert S, Schultz J, Bork P (2006) SMART 5: domains in the context of genomes and networks. Nucleic Acids Res 34:257–260
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W et al (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Johansson MW (1999) Cell adhesion molecules in invertebrate immunity. Dev Comp Immunol 23(4–5):303–315
Padhi A, Verghese B (2008) Detecting molecular adaptation at individual codons in the pattern recognition receptor, lipopolysaccharide- and β-1,3-glucan-binding protein of decapods. Fish Shellfish Immunol 24:638–648
Roux MM, Pain A, Klimpel KR, Dhar AK (2002) The lipopolysaccharide and beta-1,3-glucan binding protein gene is up-regulated in white spot virus infected shrimp, Penaeus stylirostris. J Virol 7140–7149
Pauchet Y, Freitak D, Heidel-Fischer HM, Heckel DG, Vogel H (2009) Immunity or digestion: glucanase activity in a glucan-binding protein family from Lepidoptera. J Biol Chem 284(4):2214–2224
Lichtenfels AJ, Lorenzi-Filho G, Guimaraes ET, Macchione M, Saldiva PH (1996) Effects of water pollution on the gills apparatus of fish. J Comp Pathol 115:47–60
Tiscar PG, Mosca F (2004) Defense mechanisms in farmed marine mollusks. Vet Res Commun 28:57–62
Chappell VL, Le LX, LaGrone L, Mileski WJ (2000) STAT proteins play a role in tumor necrosis factor alpha gene expression. Shock 14:400–403
Marrack P, Kappler J (1988) T cells can distinguish between allogeneic major histocompatibility complex products on different cell types. Nature 332:840–843
Acknowledgments
We are grateful to all the laboratory members for their technical advice and helpful discussions. This research was supported by the major science and technology projects of Guangdong (A200701C02; 2008A020100004), national sci-tech platform projects (2005DKA30470) and central institutes of public welfare projects (ZD-02; 2009TS23).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dianchang Zhang and Jianjun Ma authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, D., Ma, J., Jiang, J. et al. Molecular characterization and expression analysis of lipopolysaccharide and β-1,3-glucan-binding protein (LGBP) from pearl oyster Pinctada fucata . Mol Biol Rep 37, 3335–3343 (2010). https://doi.org/10.1007/s11033-009-9920-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9920-9