Abstract
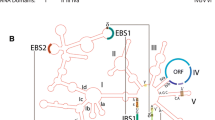
GNRA tetraloops, found in high frequency in natural RNAs, make loop-receptor interactions, stabilizing the tertiary structure of Group I introns, a class of small RNAs. Analyzing 230 Group I introns, to study the distribution and sequence pattern of the GNRA tetraloops, we suggest that these features reflect the ancestral nature of these catalytic molecules, in a prebiotic RNA world. The adenosine rich GNRA tetraloops would have interacted with each other through long range RNA–RNA interactions to form higher order structures forming potential sites that render the propensity for the short RNAs to bind to metal ions from the prebiotic pool, aiding them to act as metalloenzymes.

Similar content being viewed by others
References
Adams PL, Stahley MR, Kosek AB, Wang J, Strobel SA (2004) Crystal structure of a self-splicing group I intron with both exons. Nature 430:45–50
Barbara-Reinhold H, Shub DA (1992) Self-splicing intron in tRNA genes of widely divergent bacteria. Nature 357:173–176
Cannone JJ, Subramanian S, Schnare MN, Collet JR, D’Souzal LM, Dul Y, Feng B, Lin N, Madabusi LV, Muller KM, Pande N, Shang Z, Yul N, Gutell R (2002) The Comparative RNA Web (CRW) Site: an online database of comparative sequence and structure information for ribosomal, intron and other RNAs. BMC Bioinformatics 3:2
Caprara MG, Lehnert V, Lambowitz AM, Westhof E (1996) A tyrosyl-tRNA synthetase recognises a conserved tRNA-like structural motif in the group I intron catalytic core. Cell 87:1135–1145
Cate JH, Gooding AR, Podell E, Zhou K, Golden BL, Szewczak AA, Kundrot CE, Cech TR, Doudna JA (1996) RNA tertiary structure mediation by adenosine platforms. Science 273:1696–1699
Chandrasekhar K, Malathi R (2003) Non-Watson Crick base pairs might stabilize RNA structural motifs in ribozymes – A comparative study of group-I intron structures. J Biosci 28: 547–555
Davies RW, Waring RB, Ray JA, Brown TA, Scazzocchio C (1982) Making the ends meet: a model for RNA splicing in fungal mitochondria. Nature 300:719–724
Doherty EA, Doudna JA (2000) Ribozyme structures and mechanisms. Annu Rev Biochem 69:597–615
Engelhardt MA, Doherty EA, Knitt DS, Doudna JA, Herschlag D (2000) The P5abc peripheral element facilitates preorganization of the Tetrahymena group I ribozyme for catalysis. Biochemistry 39(10):2639–2651
Golden BL, Gooding AR, Podell ER, Cech TR (1998) A preorganized active site in the crystal structure of the Tetrahymena ribozyme. Science 282:259–264
Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S (1983) The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35:849
Jackson SA, Cannone JJ, Lee JC, Gutell RR, Woodson SA (2002) Distribution of rRNA introns in the three dimensional structure of the ribosome. J Mol Biol 323:35–52
Jaeger L, Michel F, Westhof E (1994) Involvement of a GNRA tetraloop in long-range tertiary interactions. J Mol Biol 236:1271–1276
Joyce GF (1991) The Rise and Fall of the RNA World. New Biol 3(4):399–407
Kruger K, Grobowski PJ, ZangAJ, Sands J, Gottschling DE, Cech TR (1982) Self-splicing RNA: autoexcision and autocyclization of the ribosoma; RNA intervening sequence of Tetrahymena. Cell 31:147–157
Kushel MG, Strickland R, Palmer JD (1990) An ancient Group I intron shared by eubacteria and chloroplasts. Science 250:1570–1573
Lehnert V, Jaeger L, Michel F, Westhof E (1996) New loop–loop tertiary interactions in self-splicing introns of subgroup IC and ID: a complete 3D model of the Tetrahymena thermophila ribozyme. Chem Biol 3:993–1009
Michel F, Westhof E (1990) Modeling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J Mol Biol 216:585–610
Michel F, Hanna M, Green R, Bartel DP, Szostak JW (1989) The guanosine binding site of the Tetrahymena ribozyme. Nature (London) 342:391–395
Michel F, Jacquier A, Dujon B (1982) Comparison of fungal mitochondrial introns reveals extensive homologies in RNA secondary structure. Biochimie 64(10):867–881
Murphy FL, Cech TR (1994) GAAA tetraloop and conserved bulge stabilize tertiary structure of a group I intron domain. J Mol Biol 236:49–63
Pan J, Woodson SA (1999) The effect of long-range loop–loop interactions on folding of the Tetrahymena self-splicing RNA. J Mol Biol 294:955–965
Paquin B, Kathe SD, Nierzwicki-Bauer SA, Shub DA (1997) Origin and evolution of group I introns in cyanobacterial tRNA genes. J Bacteriol 179:6798–6806
Peyman A (1994) P2 functions as a spacer in the Tetrahymena ribozyme. Nucl Acids Res 22:1383–1388
Russell R, Millett LS, Tate MW, Kwok LW, Nakatani B, Gruner SM, Mochrie VP, Dniach S, Herschlag D, Pollack L (2002) Rapid compaction during RNA folding. Proc Natl Acad Sci USA 99:4266–4271
Simon D, Fewer D, Friedl T, Bhattacharya D (2003) Phylogeny and self-splicing ability of the plastid tRNA-Leu group I Intron. J Mol Evol 57:710–720
Tanner MA, Cech TR (1996) Activity and Thermostability of the Small Self-splicing Group I Intron in the Pre-tRNAIle of the Purple Bacterium Azoarcus. RNA 2:74–83
Treiber DK, Williamson JR (2001) Beyond kinetic traps in RNA folding. Curr Opin Struct Biol 11:309–314
Woese CR, Winker S, Gutell RR (1990) Architecture of ribosomal RNA: Constraints on the sequence of tetra-loops. Proc Natl Acad Sci USA 87:8467–8471
Xu MQ, Kathe SD, Goodrich-Blair H, Nierzwicki-Bauer SA, Shub DA (1990) Bacterial origin of a chloroplast intron: conserved self-splicing group I introns in cyanobacteria. Science 250:1566–1570
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prathiba, J., Malathi, R. Group I introns and gnra tetraloops: remnants of ‘The RNA world’?. Mol Biol Rep 35, 239–249 (2008). https://doi.org/10.1007/s11033-007-9076-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-007-9076-4