Abstract
In trying to develop new anticancer agents, a series of sulfonylhydrazones were synthesized. All synthesized compounds were checked for identity and purity using elemental analysis, TLC and HPLC and were characterized by their melting points, FT-IR and NMR spectral data. All synthesized compounds were evaluated for their cytotoxic activity against prostate cancer (PC3), breast cancer (MCF-7) and L929 mouse fibroblast cell lines. Among them, N′-[(2-chloro-3-methoxyphenyl)methylidene]-4-methylbenzenesulfonohydrazide (3k) showed the most potent anticancer activity against both cancer cells with good selectivity (IC50 = 1.38 μM on PC3 with SI = 432.30 and IC50 = 46.09 μM on MCF-7 with SI = 12.94). Further investigation confirmed that 3k displayed morphological alterations in PC3 and MCF-7 cells and promoted apoptosis through down-regulation of the Bcl-2 and upregulation of Bax expression. Additionally, compound 3k was identified as the most potent COX-2 inhibitor (91% inhibition) beside lower COX-1 inhibition. Molecular docking of the tested compounds represented important binding modes which may be responsible for their anticancer activity via inhibition of the COX-2 enzyme. Overall, the lead compound 3k deserves further development as a potential anticancer agent.
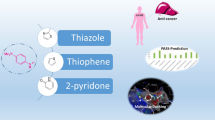
Graphic abstract
Sulfonylhydrazones was synthesized and N′-[(2-chloro-3-methoxyphenyl)methylidene]-4- methylbenzenesulfonohydrazide (3k) was identified as the most potent anticancer agent and COX-2 inhibitor. In addition, this compound docked inside the active site of COX-2 succesfully.










Similar content being viewed by others
References
Carmichael J (1994) Cancer chemotherapy: identifying novel anticancer drugs. Br Med J 308:1288–1290. https://doi.org/10.1136/bmj.308.6939.1288
Hunter AM, Lacasse ÆEC, Korneluk ÆRG (2007) The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis 12:1543–1568. https://doi.org/10.1007/s10495-007-0087-3
Ashe PC, Berry MD (2003) Apoptotic signaling cascades. Prog Neuropsychopharmacol Biol Psychiatry 308:199–214. https://doi.org/10.1016/S0278-5846(03)00016-2
Kumar R, Herbert PE, Warrens AN (2005) An introduction to death receptors in apoptosis. Int J Surg 3:268–277. https://doi.org/10.1016/j.ijsu.2005.05.002
Elumalai P, Gunadharini DN, Senthilkumar K et al (2012) Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol Lett 215:131–142. https://doi.org/10.1016/j.toxlet.2012.10.008
Sfanos KS, De Marzo AM (2014) Prostate cancer and inflammation: the evidence. Histopathology 60:199–215. https://doi.org/10.1111/j.1365-2559.2011.04033.x
Morrow RJ, Etemadi N, Yeo B, Ernst M (2017) Challenging a misnomer? The role of inflammatory pathways in inflammatory breast cancer. Mediators Inflamm 2017:4754827. https://doi.org/10.1155/2017/4754827
Liu B, Qu L, Yan S (2015) Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int 15:106. https://doi.org/10.1186/s12935-015-0260-7
Harris RE, Casto BC, Harris ZM (2014) Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J Clin Oncol 5:677–692. https://doi.org/10.5306/wjco.v5.i4.677
Gupta S, Srivastava M, Ahmad N et al (2000) Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate 42:73–78. https://doi.org/10.1002/(SICI)1097-0045(20000101)42:1%3c73:AID-PROS9%3e3.0.CO;2-G
Maekawa M, Sugan K, San H et al (1998) Increased expression of cyclooxygenase-2 to -1 in human colorectal cancers and adenomas, but not in hyperplastic polyps. Jpn J Clin Oncol 28:421–426. https://doi.org/10.1093/jjco/28.7.421
Meric J-B, Rottey S, Olaussen K et al (2006) Cyclooxygenase-2 as a target for anticancer drug development. Crit Rev Oncol Hematol 59:51–64. https://doi.org/10.1016/j.critrevonc.2006.01.003
Rollas S, Küçükgüzel SG (2007) Biological activities of hydrazone derivatives. Molecules 12:1910–1939. https://doi.org/10.3390/12081910
Aydın S, Kaushik-basu N, Arora P et al (2013) Microwave assisted synthesis of some novel Flurbiprofen hydrazide- hydrazones as anti-HCV NS5B and anticancer agents. Marmara Pharm J 17:26–34. https://doi.org/10.12991/201317389
Çıkla P, Özsavcı D, Bingöl-Özakpınar Ö et al (2013) Synthesis, cytotoxicity, and pro-apoptosis activity of etodolac hydrazide derivatives as anticancer agents. Arch Pharm (Weinheim) 346:367–379. https://doi.org/10.1002/ardp.201200449
Küçükgüzel ŞG, Koç D, Çıkla-Süzgün P et al (2015) Synthesis of tolmetin hydrazide-hydrazones and discovery of a potent apoptosis inducer in colon cancer cells. Arch Pharm (Weinheim) 348:730–742. https://doi.org/10.1002/ardp.201500178
Şenkardeş S, Kaushik-basu N, Durmaz İ et al (2016) Synthesis of novel diflunisal hydrazide hydrazones as anti-hepatitis C virus agents and hepatocellular carcinoma inhibitors. Eur J Med Chem 108:301–308. https://doi.org/10.1016/j.ejmech.2015.10.041
Tatar E, Şenkardeş S, Sellitepe E et al (2016) Synthesis, and prediction of molecular properties and antimicrobial activity of some acylhydrazones derived from N-(arylsulfonyl) methionine. Turk J Chem 40:510–534. https://doi.org/10.3906/kim-1509-21
Siemann S, Evanoff DP, Marrone L et al (2002) N-Arylsulfonyl hydrazones as inhibitors of IMP-1 metallo-β-lactamase. Antimicrob Agents Chemother 46:2450–2457. https://doi.org/10.1128/aac.46.8.2450-2457.2002
Gao Z, Lv M, Li Q, Xu H (2015) Synthesis of heterocycle-attached methylidenebenzenesulfonohydrazones as antifungal agents. Bioorg Med Chem Lett 25:5092–5096. https://doi.org/10.1016/j.bmcl.2015.10.017
Begum F, Almandil NB, Lodhi MA et al (2019) Synthesis and urease inhibitory potential of benzophenone sulfonamide hybrid in vitro and in silico. Bioorg Med Chem 27:1009–1022. https://doi.org/10.1016/j.bmc.2019.01.043
Mobasher S, Abid A, Amna H et al (2017) Sulfonyl hydrazones derived from 3-formylchromone as non-selective inhibitors of MAO-A and MAO-B:Synthesis, molecular modelling and in silico ADME evaluation. Bioorg Chem 75:291–302. https://doi.org/10.1016/j.bioorg.2017.10.001
Hayakawa M, Kawaguchi K, Kaizawa H et al (2007) Synthesis and biological evaluation of sulfonylhydrazone- substituted imidazo [1,2-a] pyridines as novel PI3 kinase p110 a inhibitors. Bioorg Med Chem 15:5837–5844. https://doi.org/10.1016/j.bmc.2007.05.070
Wei D, Pan Y, Wang H et al (2018) Synthesis of substituted aromatic heterocyclic sulfonyl hydrazone compounds and in vitro anti-hepatoma activity: preliminary results. Eur Rev Med Pharmacol Sci 22:4720–4729. https://doi.org/10.26355/eurrev_201807_15532
George RF (2018) Facile synthesis of simple 2-oxindole-based compounds with promising antiproliferative activity. Future Med Chem 10:269–282. https://doi.org/10.4155/fmc-2017-0148
Korcz M, Franciszek S, Bednarski PJ, Kornicka A (2018) Cytotoxic properties of novel quinoline-3-carbaldehyde hydrazones bearing a 1,2,4-triazole or benzotriazole moiety. Molecules 23:1497. https://doi.org/10.3390/molecules23061497
Yu Y, Huang W, Chen Y et al (2016) Calcium carbide as the acetylide source: transition-metal-free synthesis of substituted pyrazoles via [1,5]-sigmatropic rearrangements. Green Chem 18:6445–6449. https://doi.org/10.1039/c6gc02776h
Katsuhiro S, Hiraku I (1987) Thermolysis of sodium salts of tosylhydrazones of some heterocyclic aldehydes in the presence of silver chromate: 1,3 N → C migration of tosyl group. Heterocycles 26:2–9. https://doi.org/10.3987/R-1987-07-1891
Kısmet K, Akay MT, Abbasoğlu O, Ercan A (2004) Celecoxib: a potent cyclooxygenase-2 inhibitor in cancer prevention. Cancer Detect Prev 28:127–142. https://doi.org/10.1016/j.cdp.2003.12.005
Hsu A, Ching T, Wang D et al (2000) The Cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2*. J Biol Chem 275:11397–11403. https://doi.org/10.1074/jbc.275.15.11397
Altaf M, Casagrande N, Mariotto E et al (2019) Potent in vitro and in vivo anticancer activity of new bipyridine and bipyrimidine gold (III) dithiocarbamate derivatives. Cancers 11:1–14. https://doi.org/10.3390/cancers11040474
Dai Z, Ma X, Kang H et al (2012) Antitumor activity of the selective cyclooxygenase-2 inhibitor, celecoxib, on breast cancer in vitro and in vivo. Cancer Cell Int 12:1–8. https://doi.org/10.1186/1475-2867-12-53
Patel MI, Subbaramaiah K, Du B et al (2007) Celecoxib inhibits prostate cancer growth: evidence of a cyclooxygenase-2-independent mechanism. Clin Cancer Res 11:1999–2007. https://doi.org/10.1158/1078-0432.CCR-04-1877
Grösch S, Tegeder I, Niederberger E et al (2001) COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J 15:2742–2744. https://doi.org/10.1096/fj.01-0299fje
Wang Z, Chen J, Liu J (2014) COX-2 Inhibitors and gastric cancer. Gastroent Res Pract 2014:132320. https://doi.org/10.1155/2014/132320
Morrow D, Daniel R, Dubois N (1998) Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res 58:362–366
Abdelazeem AH, Gouda AM, Omar HA, Tolba MF (2014) Design, synthesis and biological evaluation of novel diphenylthiazole-based cyclooxygenase inhibitors as potential anticancer agents. Bioorg Chem 57:132–141. https://doi.org/10.1016/j.bioorg.2014.10.001
Murakami M (2011) Lipid mediators in life science. Exp Anim 60:7–20. https://doi.org/10.1538/expanim.60.7
Zhao YH, Abraham MH, Le J et al (2015) Rate-limited steps of human oral absorption and QSAR studies. Pharm Res 19:1446–1457. https://doi.org/10.1023/A:1020444330011
Daina A, Zoete V (2016) A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 11:1117–1121. https://doi.org/10.1002/cmdc.201600182
Neumann DM, Cammarata A, Backes G et al (2015) Synthesisand anti fungal activity of substituted 2,4,6-pyrimidinetrione carbalde hydehydrazones. Bioorg Med Chem 22:813–826. https://doi.org/10.1016/j.bmc.2013.12.010
Murtaza S, Shamim S, Kousar N et al (2015) Synthesis, biological investigation, calf thymus DNAbinding and docking studies of the sulfonyl hydrazides and their derivatives. J Mol Struct 1107:99–108. https://doi.org/10.1016/j.molstruc.2015.11.046
Morris GM, Huey R, Lindstrom W et al (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791. https://doi.org/10.1002/jcc.21256
Rimon G, Sidhu RS, Lauver DA et al (2010) Coxibs interfere with the action of aspirin by binding tightly to one monomer of cyclooxygenase-1. PNAS 107:28–33. https://doi.org/10.1073/pnas.0909765106
Wang JL, Limburg D, Graneto MJ et al (2010) The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: the second clinical candidate having a shorter and favorable human half-life. Bioorg Med ChemLett 20:7159–7163. https://doi.org/10.1016/j.bmcl.2010.07.054
Trott O, Olson AJ (2009) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Çoruh I, Çevik Ö, Yelekçi K et al (2018) Synthesis, anticancer activity, and molecular modeling of etodolac-thioether derivatives as potentmethionine aminopeptidase (type II) inhibitors. Arch Pharm (Weinheim) 351:e1700195. https://doi.org/10.1002/ardp.201700195
Acknowledgements
This work was supported by the Research Fund of Marmara University, Project Number: SAG-K-120917-0495.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Şenkardeş, S., Han, M.İ., Kulabaş, N. et al. Synthesis, molecular docking and evaluation of novel sulfonyl hydrazones as anticancer agents and COX-2 inhibitors. Mol Divers 24, 673–689 (2020). https://doi.org/10.1007/s11030-019-09974-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-09974-z