Abstract
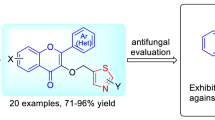
A series of novel Mannich base derivatives of flavone containing benzylamine moiety was synthesized using the Mannich reaction. The results of antifungal activity are not ideal, but its antifungal effect has a certain increase compared to flavonoids. After that, four bacteria were used to test antibacterial experiments of these compounds; compound 5g (MIC = 0.5, 0.125 mg/L) showed significant inhibitory activity against Staphylococcus aureus and Salmonella gallinarum compared with novobiocin (MIC = 2, 0.25 mg/L). Compound 5s exhibited broad spectrum antibacterial activity (MIC = 1, 0.5, 2, 0.05 mg/L) against four bacteria. The selected compounds 5g and 5s exhibit potent inhibition against Topo II and Topo IV with IC50 values (0.25–16 mg/L). Molecular docking model showed that the compounds 5g and 5s can bind well to the target by interacting with amino acid residues. It will provide some valuable information for the commercial antibacterial agents.
Graphical Abstract





Similar content being viewed by others
References
Tanaka Y, Sasaki N, Ohmiya A (2010) Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J 54:733–749. https://doi.org/10.1111/j.1365-313X.2008.03447.x
Havsteen BH (2002) The biochemistry and medical significance of the flavonoids. Pharmacol Therapeut 96:67. https://doi.org/10.1016/S0163-7258(02)00298-X
Shaw LJ, Morris P, Hooker JE (2010) Perception and modification of plant flavonoid signals by rhizosphere microorganisms. Environ Microbiol 8:1867–1880. https://doi.org/10.1111/j.1462-2920.2006.01141.x
Sakihama Y, Cohen MF, Grace SC, Yamasaki H (2002) Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology 177:67–80. https://doi.org/10.1016/S0300-483X(02)00196-8
Friedman M (2010) Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol Nutr Food Res 51:116–134. https://doi.org/10.1002/mnfr.200600173
Cushnie TPT, Lamb AJ (2005) Errata for “Antimicrobial activity of flavonoids” [Int. J. Antimicrob. Agents 26 (2005) 343–356]. Int J Antimicrob Ag 27:181
Echeverría J, Opazo J, Mendoza L, Urzúa A, Wilkens M (2017) Structure–activity and lipophilicity relationships of selected antibacterial natural flavones and flavanones of Chilean Flora. Molecules 22:608. https://doi.org/10.3390/molecules22040608
Tair A, Weiss E-K, Palade LM, Loupassaki S, Makris DP, Ioannou E et al (2014) Origanum species native to the island of Crete: in vitro antioxidant characteristics and liquid chromatography–mass spectrometry identification of major polyphenolic components. Nat Prod Res 28:1284–1287. https://doi.org/10.1080/14786419.2014.896011
Jadoon S, Karim S, Bin Asad MHH, Akram MR, Khan AK, Malik A et al (2015) Anti-aging potential of phytoextract loaded-pharmaceutical creams for human skin cell longetivity. Oxid Med Cell Longev. https://doi.org/10.1155/2015/709628
Xia JF, Gao JJ, Inagaki Y, Kokudo N, Nakata M, Tang W (2013) Flavonoids as potential anti-hepatocellular carcinoma agents: recent approaches using HepG2 cell line. Drug Discov Therapeut 7:1–8. https://doi.org/10.5582/ddt.2013.ddt.v7.1.1
Rao YJ, Sowjanya T, Thirupathi G, Kotapalli SS (2018) Synthesis and biological evaluation of novel flavone/triazole/benzimidazole hybrids and flavone/isoxazole-annulated heterocycles as antiproliferative and antimycobacterial agents. Mol Divers. https://doi.org/10.1007/s11030-018-9833-4
Kagechika H, Kawachi E, Hashimoto Y, Shudo K (1989) Retinobenzoic acids. 2. Structure-activity relationships of chalcone-4-carboxylic acids and flavone-4′-carboxylic acids. J Med Chem 32:834. https://doi.org/10.1021/jm00124a016
Zwaagstra ME, Timmerman H, Abdoelgafoer RS, Zhang MQ (1996) Synthesis of carboxylated flavonoids as new leads for LTD4 antagonists. Eur J Med Chem 31:861–874. https://doi.org/10.1016/S0223-5234(97)89849-2
Krauss J, Stadler M, Bracher F (2017) Synthesis and structure–activity relationships of novel benzylamine-type antifungals as butenafine-related antimycotics. Arch Pharm. https://doi.org/10.1002/ardp.201600342
Kaya B, Saglik BN, Levent S, Ozkay Y, Kaplancikli ZA (2016) Synthesis of some novel 2-substituted benzothiazole derivatives containing benzylamine moiety as monoamine oxidase inhibitory agents. J Enzyme Inhib Med Chem 31:1654–1661. https://doi.org/10.3109/14756366.2016.1161621
Sui Z, Nguyen VN, Altom J, Fernandez J, Hilliard JJ, Bernstein JI et al (1999) Synthesis and topoisomerase inhibitory activities of novel aza-analogues of flavones 1. Eur J Med Chem 34:381–387. https://doi.org/10.1016/S0223-5234(99)80087-7
Bernard FX, Sablé S, Cameron B, Provost J, Desnottes JF, Crouzet J et al (1997) Glycosylated flavones as selective inhibitors of topoisomerase IV. Antimicrob Agents Ch 41:992–998
Sun X, Hu CQ, Huang XD, Dong JC (2003) Mannich reaction of baicalein. Chin J Org Chem 23:81–85
Helgren TR, Sciotti RJ, Lee P, Duffy S, Avery VM, Igbinoba O et al (2015) The synthesis, antimalarial activity and CoMFA analysis of novel aminoalkylated quercetin analogs. Bioorg Med Chem Lett 25:327–332. https://doi.org/10.1016/j.bmcl.2014.11.039
Morales JC, Zurita D, Penadés S (1998) Carbohydrate–carbohydrate interactions in water with glycophanes as model systems. J Org Chem 63:9212–9222
Lehmann PF (1999) P.R. Murray, E.J. Baron, M.A. Pfaller, F.C. Tenover and R.H. Yolken, eds. Manual of clinical microbiology, 7th ed. Mycopathologia 146:107–108. https://doi.org/10.1023/a:1007025717379
Jorgensen JH (1993) NCCLS methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard. Infect Dis Clin North Am 7:393–409
Aragade P, Maddi V, Khode S, Palkar M, Ronad P, Mamledesai S et al (2009) Synthesis and antibacterial activity of a new series of 3–3-(substituted phenyl)-1-isonicotinoyl-1h-pyrazol-5-yl-2h-chromen-2-one derivatives. Arch Pharm 342:361–366. https://doi.org/10.1002/ardp.200800156
Sato K, Inoue Y, Fujii T, Aoyama H, Inoue M, Mitsuhashi S (1986) Purification and properties of DNA gyrase from a fluoroquinolone-resistant strain of Escherichia coli. Antimicrob Agents Ch 30:777–780
Peng H, Marians KJ (1993) Escherichia coli topoisomerase IV. Purification, characterization, subunit structure, and subunit interactions. J Biol Chem 268:24481–24490
Dai YJ, Wang QA, Zhang XL, Jia SR, Zheng H, Feng DC et al (2010) Molecular docking and QSAR study on steroidal compounds as aromatase inhibitors. Eur J Med Chem 45:5612–5620. https://doi.org/10.1016/j.ejmech.2010.09.011
Wu G, Robertson DH, Iii CLB, Vieth M (2003) Detailed analysis of grid-based molecular docking: a case study of CDOCKER—a CHARMm-based MD docking algorithm. J Co Ch 24:1549–1562. https://doi.org/10.1002/jcc.10306
Acknowledgements
This work was supported by The Basic Science Research Fund Program of ICBR (1632017005), Natural Science Foundation of Education Committee of Anhui Province (KJ2018A0162) and National Key R&D Program of China (Project No. 2017YFD0600805). We are grateful to Prof. Hai-Liang Zhu (State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, Nanjing) for computational molecular docking assistance.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lv, XH., Liu, H., Ren, ZL. et al. Design, synthesis and biological evaluation of novel flavone Mannich base derivatives as potential antibacterial agents. Mol Divers 23, 299–306 (2019). https://doi.org/10.1007/s11030-018-9873-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9873-9