Abstract
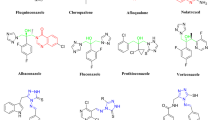
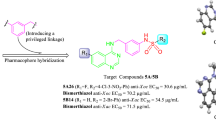
A series of quinazoline derivatives containing a 1,3,4-oxadiazole moiety were synthesized and evaluated for their antibacterial activities against Xanthomonas axonopodis pv. citri (Xac) and Ralstonia solanacearum (Rs). Antibacterial bioassays indicated that most of target compounds exhibited significant antibacterial activities against Xac and Rs in vitro. Strikingly, compounds 6d–6i, 6m–6r and 6u–6x showed antibacterial activity against Xac, with \(\hbox {EC}_{50}\) values ranging from 14.42 to 38.91 \(\upmu \)g/mL, which are better than that of bismerthiazol (39.86 \(\upmu \)g/mL). Based on the antibacterial activity against Xac, comparative molecular filed analysis and comparative molecular similarity index analysis models were generated to investigate the structure-activity relationship of title compounds against Xac. The analytical results indicated that the above models exhibited good predictive accuracy and could be used as practical tools for guiding the design and synthesis of more potent quinazoline derivatives containing a 1,3,4-oxadiazole moiety.







Similar content being viewed by others
References
Wang X, Li P, Li Z, Yin J, He M, Xue W, Chen Z, Song B (2013) Synthesis and bioactivity evaluation of novel arylimines containing a 3-aminoethyl-2-[(\(p\)-trifluoromethoxy)anilino] -4(\(3H)\)-quinazolinone moiety. J Agric Food Chem 61:9575–9582. https://doi.org/10.1021/jf4031939
Wang PY, Zhou L, Zhou J, Wu ZB, Xue W, Song BA, Yang S (2016) Synthesis and antibacterial activity of pyridinium-tailored 2,5-substituted-1,3,4-oxadiazole thioether/sulfoxide/sulfone derivatives. Bioorg Med Chem Lett 26:1214–1217. https://doi.org/10.1016/j.bmcl.2016.01.029
Xu WM, Han FF, He M, Hu DY, He J, Yang S, Song BA (2012) Inhibition of tobacco bacterial wilt with sulfone derivatives containing an 1,3,4-oxadiazole moiety. J Agric Food Chem 60:1036–1041. https://doi.org/10.1021/jf203772d
Halby L, Menon Y, Rilova E, Pechalrieu D, Massion V, Faux C, Bouhlei MA, David-Cordonnier MH, Novosad N, Aussagues Y, Samson A, Lacroix L, Ausseil F, Fleury L, Guianvarch D, Ferroud C, Arimondo PB (2017) Rational design of bisubstrate-type analogues as inhibitors of DNA methyltransferases in cancer cells. J Med Chem 60:4665–4679. https://doi.org/10.1021/acs.jmedchem.7b00176
Venkatesham A, Saudi M, Kaptein S, Neyts J, Rozenski J, Froeyen M, Aerschot AV (2017) Aminopurine and aminoquinazoline scaffolds for development of potential dengue virus inhibitors. Eur J Med Chem 126:101–109. https://doi.org/10.1016/j.ejmech.2016.10.008
Abuelizz HA, Dib RE, Marzouk M, Anouar EH, Maklad YA, Attia HN, Al-Salahi R (2017) Molecular docking and anticonvulsant activity of newly synthesized quinazoline derivatives. Molecules 22:1094–1107. https://doi.org/10.3390/molecules22071094
Gilson PR, Tan C, Jarman KE, Lowes KN, Curtis JM, Nguyen W, Rago AED, Bullen HE, Prinz B, Duffy S, Baell JB, Hutton CA, Subroux HJ, Crabb BS, Avery VM, Cowman AF, Sleebs BE (2017) Optimization of 2-anilino 4-amino substituted quinazolines into potent antimalarial agents with oral in vivo activity. J Med Chem 60:1171–1188. https://doi.org/10.1021/acs.jmedchem.6b01673
Hu J, Zhang Y, Dong L, Wang Z, Chen L, Liang D, Shi D, Shan X, Liang G (2015) Design, synthesis, and biological evaluation of novel quinazoline derivatives as anti-inflammatory agents against lipopolysaccharide-induced acute lung injury in rats. Chem Biol Drug Des 85:672–684. https://doi.org/10.1111/cbdd.12454
Mohamed T, Rao PP (2017) 2,4-Disubstituted quinazolines as amyloid-\(\beta \) aggregation inhibitors with dual cholinesterase inhibition and antioxidant properties: development and structure-activity relationship (SAR) studies. Eur J Med Chem 126:823–843. https://doi.org/10.1016/j.ejmech.2016.12.005
Yu G, Li Z, Tang L, Xiong Q (2014) Synthesis and evaluation of 2,4-disubstituted quinazoline derivatives with potent anti-angiogenesis activities. Molecules 19:8916–8932. https://doi.org/10.3390/molecules19078916
Desroches J, Kieffer C, Primas N, Hutter S, Gellis A, Ei-Kashef H, Rathelot P, Verhaeghe P, Azas N, Vanelle P (2017) Discovery of hit-molecules targeting plasmodium falciparum through a global SAR study of the 4-substituted-2-trichloromethylquinazoline antiplasmodial scaffold. Eur J Med Chem 125:68–86. https://doi.org/10.1016/j.ejmech.2016.09.029
Patel AB, Chikhalia KH, Kumari P (2015) Access to antimycobacterial and anticancer potential of some fused quinazolines. Res Chem Intermed 41:4439–4455. https://doi.org/10.1007/s11164-014-1542-8
Ma Y, Liu F, Yan K, Song BA, Yang S, Hu DY, Jin LH, Xue W (2008) Synthesis and antifungal bioactivity of 6-bromo-4-alkylthioquinazoline derivatives. Chin J Organ Chem 28:1268–1272
Luo H, Liu J, Jin L, Hu D, Chen Z, Yang S, Wu J, Song B (2013) Synthesis and antiviral bioactivity of novel (1E, 4E)-1-aryl-5-(2-(quinazolin-4-yloxy)phenyl)-1,4-pentadien-3-one derivatives. Eur J Med Chem 63:662–669. https://doi.org/10.1016/j.ejmech.2013.02.035
Wan Z, Hu D, Li P, Xie D, Gan X (2015) Synthesis, antiviral bioactivity of novel 4-thioquinazoline derivatives containing chalcone moiety. Molecules 20:11861–11874. https://doi.org/10.3390/molecules200711861
Wu Z, Zhang J, Chen J, Pan J, Zhao L, Liu D, Zhang A, Chen J, Hu D, Song B (2017) Design, synthesis, antiviral bioactivity and three-dimensional quantitative structure-activity relationship study of novel ferulic acid ester dderivatives containing quinazoline moiety. Pest Manag Sci 73:2079–2089. https://doi.org/10.1002/ps.4579
Xiao H, Li P, Hu D, Song BA (2014) Synthesis and anti-TMV activity of novel \(\beta \)-amino acid ester derivatives containing quinazoline and benzothiazole moieties. Bioorg Med Chem Lett 24:3452–3454. https://doi.org/10.1016/j.bmcl.2014.05.073
Xu GF, Song BA, Bhadury PS, Yang S, Zhang PQ, Jin LH, Xue W, Hu DY, Lu P (2007) Synthesis and antifungal activity of novel S-substituted 6-fluoro-4-alkyl(aryl)thioquinazoline derivatives. Bioorg Med Chem 15:3768–3774. https://doi.org/10.1016/j.bmc.2007.03.037
Yang L, Bao XP (2017) Synthesis of novel 1,2,4-triazole derivatives containing the quinazolinylpiperridinyl moiety and N-(substituted phenyl)acetamide group as efficient bactericides against the phytopathogenic bacterium Xanthomonas oryzae pv. oryzae. RSC Adv 7:34005–34011. https://doi.org/10.1039/c7ra04819j
Bhattacharyya J, Banerjee H, Bhattacharyya A (2003) Photodecomposition of acaricide, fenazaquin, in aqueous alcoholic solution. J Agric Food Chem 51:4013–4016. https://doi.org/10.1021/jf034034c
Dawson WAJM, Bateman GL (2000) Sensitivity of fungi from cere al roots to fluquinconazole and their suppressiveness towards take-all on plants with or without fluquinconazole seed treatment in a controlled environment. Plant Pathol 49:477–486. https://doi.org/10.1046/j.1365-3059.2000.00479.x
Wang PY, Zhou L, Zhou J, Fang HS, Wu ZB, Xue W, Song BA, Yang S (2017) Potent antibacterial agents: pyridinium-functionalized amphiphiles bearing 1,3,4-oxadiazole scaffolds. Chem Pap 71:1013–1018. https://doi.org/10.1007/s11696-016-0021-7
Xu W, He J, He M, Han F, Chen X, Pan Z, Wang J, Tong M (2011) Synthesis and antifungal activity of novel sulfone derivatives containing 1,3,4-oxadiazole moieties. Molecules 16:9129–9141. https://doi.org/10.3390/molecules16119129
Chen X, Gan X, Chen J, Chen Y, Wang Y, Hu D, Song B (2017) Synthesis and nematicidal activity of novel 1,3,4-oxadiazole (thiadiazole) thioether derivatives containing trifluorobuten moiety. Chin J Organ Chem 37:2343–2351. https://doi.org/10.6023/cioc201703022
Gan X, Hu D, Li P, Wu J, Chen X, Xue W, Song B (2016) Design, synthesis, antiviral activity and three-dimensional quantitative structure-activity relationship study of novel 1,4-pentadien-3-one derivatives containing the 1,3,4-oxadiazole moiety. Pest Manag Sci 72:534–543. https://doi.org/10.1002/ps.4018
Liu ZM, Yang GF, Qin XH (2001) Synthesis and biological activities of novel diheterocyclic compounds containing 1,2,4-triazolo[1,5-\(\alpha \)]pyrimidine and 1,3,4-oxadizole. J Chem Technol Biotechnol 76:1154–1158. https://doi.org/10.1002/jctb.500
Li P, Yin J, Xu W, Wu J, He M, Hu D, Yang S, Song B (2013) Synthesis, antibacterial activities, and 3D-QSAR of sulfone derivatives containing 1,3,4-oxadiazole moiety. Chem Biol Drug Des 82:546–556. https://doi.org/10.1111/cbdd.12181
Shi L, Li P, Wang W, Gao M, Wu Z, Song X, Hu D (2015) Antibacterial activity and mechanism of action of sulfone derivatives containing 1,3,4-oxadiazole moieties on rice bacterial leaf blight. Molecules 20:11660–11675. https://doi.org/10.3390/molecules200711660
Li P, Shi L, Yang X, Yang L, Chen XW, Wu F, Shi QC, Xu WM, He M, Hu DY, Song BA (2014) Design, synthesis, and antibacterial activity against rice bacterial leaf blight and leaf streak of 2,5-substituted-1,3,4-oxadiazole/thiadiazole sulfone derivative. Bioorg Med Chem Lett 24:1677–1680. https://doi.org/10.1016/j.bmcl.2004.02.060
Su S, Zhou X, Liao G, Qi P, Jin L (2017) Synthesis and antibacterial evaluation of new sulfone derivatives containing 2-aroxymethyl-1,3,4-oxadiazole/thiadiazole moiety. Molecules 22:64–81. https://doi.org/10.3390/molecules22010064
Wang PY, Chen L, Zhou J, Fang HS, Wu ZB, Song BA, Yang S (2017) Synthesis and bioactivities of 1-ary-4-hydroxy-1H-pyrrol-2(5H)-one derivatives bearing 1,3,4-oxadiazole moiety. J Saudi Chem Soc 21:315–323. https://doi.org/10.1016/j.jscs.2016.10.002
Zhou L, Wang PY, Zhou J, Shao WB, Fang HS, Wu ZB, Yang S (2017) Antimicrobial activities of pyridinium-tailored pyrazoles bearing 1,3,4-oxadiazole scaffolds. J Saudi Chem Soc 21:852–860. https://doi.org/10.1016/j.jscs.2017.04.005
Wang X, Zhong X, Zhu X, Wang H, Li Q, Zhang J, Ruan X, Xue W (2017) Synthesis and antibacterial activity of oxime ester derivatives containing 1,2,4-triazole or 1,3,4-oxadiazole moiety. Chem Pap 71:1953–1960. https://doi.org/10.1007/s11696-017-0189-5
Golbraikh A, Tropsha A (2002) Beware of \(\text{ q }^{2}\). J Mol Graph Model 20:269–276. https://doi.org/10.1016/s1093-3263(01)00123-1
Huang XY, Shan ZJ, Zhai HL, Li LN, Zhang XY (2011) Molecular design of anticancer drug leads based on three-dimensional quantitative structure-activity relationship. J Chem Inf Model 51:1999–2006. https://doi.org/10.1021/ci2002236
Amin EA, Welsh W (2006) Highly predictive CoMFA and CoMSIA models for two series of stromelysin-1 (MMP-3) inhibitors elucidate S1’ and S1–S2’ binding modes. J Chem Inf Model 46:1775–1783. https://doi.org/10.1021/ci060089d
Cruciani G, Baroni M, Clementi S, Costantino G, Riganelli D, Skagerberg B (1992) Predictive ability of regression models. Part I: standard deviation of prediction errors (SDEP). J Chemom 6:335–346. https://doi.org/10.1002/cem.1180060604
Baroni M, Clementi S, Cruciani G, Costantino G, Riganelli D, Oberrauch E (1992) Predictive ability of regression models. Part II: selection of the best predictive PLS model. J Chemom 6:347–356. https://doi.org/10.1002/cem.1180060605
Acknowledgements
The authors gratefully acknowledge grants from the National Key Research and Development Program of China (No. 2017YFD0200506), the National Nature Science Foundation of China (No. 21462012) and the Special Fund for Outstanding Scientific and Technological Candidates of Guizhou Province (No. 2015035).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, X., Yan, J., Wang, M. et al. Synthesis and three-dimensional quantitative structure-activity relationship study of quinazoline derivatives containing a 1,3,4-oxadiazole moiety as efficient inhibitors against Xanthomonas axonopodis pv. citri. Mol Divers 22, 791–802 (2018). https://doi.org/10.1007/s11030-018-9837-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9837-0