Abstract
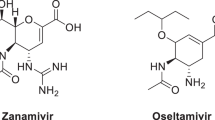
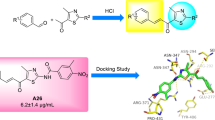
Two series of novel 2-thiazolylhydrazone derivatives were designed and synthesized via one-pot reaction of benzaldehyde derivatives, \(\alpha \)-haloketones and thiosemicarbazide. The structures of compounds 1 and 2 were characterized by \(^{1}\hbox {H}\) NMR and \(^{13}\hbox {C}\) NMR, and compound 1g was further confirmed by X-ray crystallography. All of the target compounds were evaluated for their NA inhibitory activity against influenza viral neuraminidase (H1N1) in vitro, and the results showed that many compounds exhibited moderate to strong inhibitory activities against influenza viral neuraminidase (H1N1). Among them, compounds 1p, 1q and 2c showed the most potent inhibitory activities with \(\hbox {IC}_{50}\) values ranging from 10.50 to \(13.75\, \upmu \hbox {g}/\hbox {mL}\). Our structure–activity relationship analysis indicated that 2-thiazolylhydrazone is an effective scaffold for NA inhibitors and that introducing an ethoxycarbonyl group to the 5-position of thiazole ring could enhance inhibitory potency. Molecular docking was performed on the most active compounds 1p and 2c to provide more insight into their mechanism of interaction.







Similar content being viewed by others
References
Williams S, Fitzner J, Merianos A, Mount A, Case-based Surveillance Evaluation Group (2014) The challenges of global case reporting during pandemic A(H1N1) 2009. Bull World Health Organ 92:60–67. doi:10.2471/BLT.12.116723
Liu D, Shi W, Shi Y, Wang D, Xiao H, Li W, Bi Y, Wu Y, Li X, Yan J, Liu W, Zhao G, Yang W, Wang Y, Ma J, Shu Y, Lei F (2013) Origin and diversity of novel avian influenza A H7N9 viruses causing human infection: phylogenetic, structural, and coalescent analyses. Lancet 381:1926–1932. doi:10.1016/S0140-6736(13)60938-1
Kayali G, Kandeil A, El-Shesheny R, Kayed AS, Maatouq AM, Cai Z, McKenzie PP, Webby RJ, Refaey SE, Kandeel A, Ali MA (2016) Avian influenza A(H5N1) virus in Egypt. Emerg Infect Dis 22:379–388. doi:10.3201/eid2203.150593
Wang Y, Ge H, Li Y, Xie Y, He Y, Xu M, Gu Q, Xu J (2014) Predicting dual-targeting anti-influenza agents using multi-models. Mol Divers 19:123–134. doi:10.1007/s11030-014-9552-4
Wu G, Yan S (2007) Prediction of mutations in H1 neuraminidases from North America influenza a virus engineered by internal randomness. Mol Divers 11:131–140. doi:10.1007/s11030-008-9067-y
The MIST Study Group (1998) Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet 352:1877–1881. doi:10.1016/S0140-6736(98)10190-3
Tashani M, Rashid H, Ridda I, Heron L, Memish ZA, Haworth E, Booy R (2013) Oseltamivir for control of influenza at mass gatherings. Curr Drug Targets 13:46–52. doi:10.2174/18715265112129990007
Gong J, Xu W, Zhang J (2007) Structure and functions of influenza virus neuraminidase. Curr Med Chem 14:113–122. doi:10.2174/092986707779313444
Robert NA (2001) Anti-influenza drugs and neuraminidase inhibitors. Prog Drug Res 56:195–237. doi:10.1007/978-3-0348-8319-1_5
Jose L, Franco M, Giulianotti MA, Welmaker GS, Houghten RA (2013) Shifting from the single to the multitarget paradigm in drug discovery. Drug Discov Today 18:495–501. doi:10.1016/j.drudis.2013.01.008
Lian W, Fang J, Li C, Pang X, Liu AL, Du GH (2015) Discovery of influenza A virus neuraminidase inhibitors using support vector machine and Naïve bayesian models. Mol Divers 20:1–13. doi:10.1007/s11030-015-9641-z
Wang GT, Chen Y, Wang S, Gentles R, Sowin T (2001) Design, synthesis, and structural analysis of influenza neuraminidase inhibitors containing pyrrolidine cores. J Med Chem 44:1192–1201. doi:10.1021/jm000468c
Von Itzstein M, Wu WY, Kok GB, Pegg MS, Dyason JC, Jin B, Van Phan T, Smythe ML, White HF, Oliver SW (1993) Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363:418–23. doi:10.1038/363418a0
Liu Y, Jing F, Xua Y, Xie Y, Shi F, Hao G, Li M, Xu W (2011) Design, synthesis and biological activity of thiazolidine-4-carboxlic acid derivatives as novel influenza neuraminidase inhibitors. Bioorg Med Chem 19:2342–2348. doi:10.1016/j.bmc.2011.02.019
Zhao J, Aisa AH (2012) Synthesis and anti-influenza activity of aminoalkyl rupestonates. Bioorg Med Chem Lett 22:2321–2325. doi:10.1016/j.bmcl.2012.01.056
Wu Z, Peng J, Hu A, Ye J, Li G (2015) Design, synthesis and neuraminidase inhibitory activity of N-(5-benzyl-4-(tert-butyl) thiazol-2-yl)benzamides. Med Chem Res 24:1–13. doi:10.1007/s00044-015-1487-5
Fang Y, Xiao M, Hu A (2016) Design, synthesis, and evaluation of 3-((4-(\(t\)-butyl)-2-(2-benzylidenehydrazinyl) thiazol-5-yl)methyl)quinolin-2(1 \(H\))-ones as neuraminidase inhibitors. Chin J Chem 34:403–411. doi:10.1002/cjoc.201500738
Lohrey L, Uehara TN, Tani S, Yamaguchi J, Humpf HU, Itami K (2014) 2,4- and 2,5-disubstituted arylthiazoles. Rapid synthesis by C–H coupling and biological evaluation. Eur J Org Chem 16:3387–3394. doi:10.1002/ejoc.201402129
Bharti SK, Singh SK (2014) Design, synthesis and biological evaluation of some novel benzylidene-2-(4-phenylthiazol-2-yl) hydrazines as potential anti-inflammatory agents. Med Chem Res 23:1004–1015. doi:10.1007/s00044-013-0708-z
Shih MH, Su YS, Wu CL (2007) Syntheses of aromatic substituted hydrazino-thiazole derivatives to clarify structural characterization and antioxidant activity between 3-arylsydnonyl and aryl substituted hydrazinothiazoles. Chem Pharm Bull 55:1126–1135. doi:10.1248/cpb.55.1126
Zhang DN, Li JT, Song YL, Liu HM, Li HY (2012) Efficient one-pot three-component synthesis of N-(4-arylthiazol-2-yl) hydrazones in water under ultrasound irradiation. Ultrason Sonochem 19:475–478. doi:10.1016/j.ultsonch.2011.10.017
Ding Q, Zhu D, Jin H, Chen J (2011) Eco-friendly one-pot synthesis of 2,4-disubstituted thiazoles by grinding under catalyst and solvent-free conditions. Phosphorus Sulfur 186:220–224. doi:10.1080/10426507.2010.492366
Ye J, Qiu S, Hu A, Sun X (2009) CN102875543
Caputto ME, Ciccarelli A, Frank F (2012) Synthesis and biological evaluation of some novel 1-indanone thiazolylhydrazone derivatives as anti-Trypanosoma cruzi agents. Eur J Med Chem 55:155–163. doi:10.1016/j.ejmech.2012.07.013
Zhao H, Huang D, Caflisch A (2012) Discovery of tyrosine kinase inhibitors by docking into an inactive kinase conformation generated by molecular dynamics. ChemMedChem 7:1983–1990. doi:10.1002/cmdc.201200331
Wang Z, Sun H, Yao X, Li D, Xu L, Li Y (2016) Comprehensive evaluation of ten docking programs on a diverse set of protein–ligand complexes: the prediction accuracy of sampling power and scoring power. Phys Chem Chem Phys 18:12964–12975. doi:10.1039/C6CP01555G
Liu AL, Cao HP, Du GH (2005) Drug screening for influenza neuraminidase inhibitors. Sci China Ser C 48:1–5. doi:10.1360/062004-69
Wang ZM, Tai CY, Mendel DB (2000) Characterization of human influenza virus variants selected in vitro in the presence of the neuraminidase inhibitor GS4071. Antivir Res 46:A60. doi:10.1016/S0166-3542(00)90411-X
Mitrasinovic PM (2009) On the structure-based design of novel inhibitors of H5N1 influenza A virus neuraminidase (NA). Biophys Chem 140:35–38. doi:10.1016/j.bpc.2008.11.004
Wallace AC, Laskowski RA, Thornton JM (1995) LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng 8:127–134. doi:10.1093/protein/8.2.127
Acknowledgements
This work was supported by Emergency Project of National Natural Science Fund (No. 21442014). The institute of Materia Medica of Chinese Academy of Medical Sciences and Peking Union Medical College provided the biological activity assays.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuan, K., Xiao, M., Tan, Y. et al. Design and one-pot synthesis of 2-thiazolylhydrazone derivatives as influenza neuraminidase inhibitors. Mol Divers 21, 565–576 (2017). https://doi.org/10.1007/s11030-017-9740-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-017-9740-0