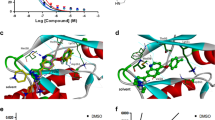
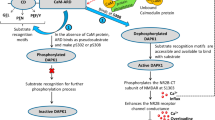
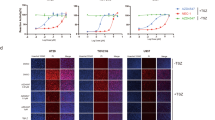
Abstract
Necroptosis or programmed necrosis is evident in various neurological disorders such as ischemic stroke. Receptor interacting serine/threonine protein kinase 3 (RIPK3) is one of the crucial targets of necroptosis and inhibition of this protein exerts neuroprotection. However, knowledge regarding the three-dimensional structure and binding site information of this protein is lacking. In the present study, structure-based in silico methods were implemented to identify the key amino acids in the RIPK3 binding site that might be responsible for ligand interactions. Further, novel RIPK3 inhibitors were identified through a dual ensemble screening strategy. Three inhibitors exhibited binding to RIPK3 in micromolar concentrations and exerted post-ischemic neuroprotection in vitro.









Similar content being viewed by others
References
Fayaz SM, Suvanish Kumar V, Rajanikant GK (2014) Necroptosis: who knew there were so many interesting ways to die? CNS Neurol Disord Drug Targets 13:42–51. doi:10.2174/18715273113126660189
Linkermann A, Hackl MJ, Kunzendorf U, Walczak H, Krautwald S, Jevnikar AM (2013) Necroptosis in immunity and ischemia reperfusion injury. Am J Transplant 13:2797–2804. doi:10.1111/ajt.12448
Mehta SL, Manhas N, Raghubir R (2007) Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 54:34–66. doi:10.1016/j.brainresrev.2006.11.003
Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ (2003) A role for tumor necrosis factor receptor-2 and receptor interacting protein in programmed necrosis and antiviral responses. J Biol Chem 278:51613–51621. doi:10.1074/jbc.M305633200
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1:489–495. doi:10.1038/82732
Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, Tran JH, Nedospasov SA, Liu ZG (2004) Tumor necrosis factor induced non-apoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem 279:10822–10828. doi:10.1074/jbc.M313141200
Ch’en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM (2011) Mechanisms of necroptosis in T cells. J Exp Med 208:633–641. doi:10.1084/jem.20110251
Goossens V, De Vos K, Vercammen D, Steemans M, Vancompernolle K, Fiers W, Vandenabeele P, Grooten J (1999) Redox regulation of TNF signaling. Biofactors 10:145–156. doi:10.1002/biof.5520100210
Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, Tran JH, Nedospasov SA, Liu ZG (2004) Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein mediated cellular reactive oxygen species accumulation. J Biol Chem 279:10822–10828. doi:10.1074/jbc.M313141200
Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES (2011) RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471:368–372. doi:10.1038/nature09857
Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR (2011) Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471:363–367. doi:10.1038/nature09852
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325:332–336. doi:10.1126/science.1172308
Vanlangenakker N, Bertrand MJ, Bogaert P, Vandenabeele P, Vanden Berghe T (2011) TNF induced necroptosis in L929 cells is tightly regulated by multiple TNFR1 complex I and II members. Cell Death Dis 2:e230. doi:10.1038/cddis.2011.111
Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG (2012) Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci USA 109:5322–5327. doi:10.1073/pnas.1200012109
Wu XN, Yang ZH, Wang XK, Zhang Y, Wan H, Song Y, Chen X, Shao J, Han J (2014) Distinct roles of RIP1–RIP3 hetero- and RIP3–RIP3 homo-interaction in mediating necroptosis. Cell Death Differ 21:1709–1720. doi:10.1038/cdd.2014.77
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Fayaz SM, Rajanikant GK (2014) Ensemble pharmacophore meets ensemble docking: a novel screening strategy for the identification of RIPK1 inhibitors. J Comput Aided Mol Des 28:779–794. doi:10.1007/s10822-014-9771-x
Darden T, York D, Pedersen L (1993) Particle mesh Ewald—an N.log(N) method for Ewald sums in large systems. J Chem Phys 98:10089–10093. doi:10.1063/1.464397
Salam NK, Nuti R, Sherman W (2009) Novel method for generating structure-based pharmacophores using energetic analysis. J Chem Inf Model 49:2356–2368. doi:10.1021/ci900212v
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749. doi:10.1021/jm0306430
Irwin JJ, Shoichet BK (2005) ZINC—a free database of commercially available compounds for virtual screening. J Chem Inf Model 45:177–182. doi:10.1021/ci049714
Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108
Fayaz SM, Rajanikant GK (2015) Ensembling and filtering: an effective and rapid in silico multitarget drug-design strategy to identify RIPK1 and RIPK3 inhibitors. J Mol Model 21:314–327. doi:10.1007/s00894-015-2855-2
Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 5:313–321. doi:10.1038/nchembio.83
Acknowledgments
This study was funded by the Department of Biotechnology, Government of India “Bioinformatics Infrastructure Facility for Biology Teaching through Bioinformatics (BIF-BTBI)” (Grant number: BT/BI/25/001/2006 dated 25/03/2011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fayaz, S.M., Suvanish Kumar, V.S., Davis, C.K. et al. Novel RIPK3 inhibitors discovered through a structure-based approach exert post-ischemic neuroprotection. Mol Divers 20, 719–728 (2016). https://doi.org/10.1007/s11030-016-9663-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-016-9663-1