Abstract
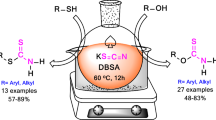
A mild, efficient, and selective protocol for the one-pot \(N\)-alkylation of sulfonamides with alcohols using triphenylphosphine and carbon tetrachloride is described. In this method, treatment of alcohols with a mixture of triphenylphosphine, carbon tetrachloride, and potassium sulfonylamide salts in refluxing anhydrous DMF furnishes the corresponding \(N\)-alkyl sulfonamides in good to excellent yields. This protocol is highly efficient for various structurally diverse alcohols and potassium sulfonylamide salts. In this paper the influence of solvents and various reagents as sources for electrophilic-halogen instead of carbon tetrachloride in combination with triphenylphosphine have been examined. This protocol demonstrates the selectivity between primary and secondary alcohols. A plausible mechanism for this protocol has been described.


Similar content being viewed by others
References
Severin S, Doye S (2007) The catalytic hydroamination of alkynes. Chem Soc Rev 36:1407–1420. doi:10.1039/B600981F
Hultzsch KC (2005) Transition metal-catalyzed asymmetric hydroamination of alkenes (AHA). Adv Synth Catal 347:367–391. doi:10.1002/adsc.200404261
Kleeman A, Engel J, Kutscher B, Reichert D (1999) Pharmaceutical substances, 3rd edn. Thieme, Stuttgart
Wilson CO, Gisvold O, Block JH (2004) In: Block JH, Beale JM (eds) Wilson and Gisvold’s textbook of organic medicinal and pharmaceutical chemistry, 11th ed. Lippincott Williams & Wilkins, Philadelphia
Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT (2003) Anticancer and antiviral sulfonamides. Curr Med Chem 10:925–953. doi:10.2174/0929867033457647
Drew J (2000) Drug discovery: a historical perspective. Science 287:1960–1964. doi:10.1126/science.287.5460.1960
Scozzafava A, Supuran CT (2000) Carbonic anhydrase and matrix metalloproteinase inhibitors: sulfonylated amino acid hydroxamates with MMP inhibitory properties act as efficient inhibitors of CA isozymes I, II, and IV, and N-hydroxysulfonamides inhibit both these zinc enzymes. J Med Chem 43:3677–3687. doi:10.1021/jm000027t
Quaal KS, Ji S, Kim YM, Closson WD, Zubieta JA (1978) Substituent effects on reductive cleavage of N-methylarene- sulfonanilides. Cleavage by sodium anthracene and electrochemically at the vitreous carbon electrode. J Org Chem 43:1311–1316. doi:10.1021/jo00401a005
Caddick S, Wilden JD, Wadman SJ, Bush HD, Judd DB (2002) A new route to sulfonamides via intermolecular radical addition to pentafluorophenyl vinylsulfonate and subsequent aminolysis. Org Lett 4:2549–2551. doi:10.1021.ol026181m
Kamal AJ, Reddy S, Bharathi EV, Dastagiri D (2008) Base-free monosulfonylation of amines using tosyl or mesyl chloride in water. Tetrahedron Lett 49:348–353. doi:10.1016/j.tetlet.2007.11.044
Zhang J, Yang CG, He C (2006) Gold(I)-catalyzed intra- and intermolecular hydroamination of unactivated olefins. J Am Chem Soc 128:1798–1799. doi:10.1021/ja053864z
Giner X, Nájera C (2008) (Triphenyl phosphite) gold (I)-catalyzed intermolecular hydroamination of alkenes and 1,3-dienes. Org Lett 10:2919–2922. doi:10.1021/ol801104w
Yang L, Xu LW, Zhou W, Gao YH, Sun W, Xia CG (2009) Zirconium-catalyzed intermolecular hydroamination of unactivated olefins. Synlett 7:1167–1171. doi:10.1055/s-0028-1088151
Caddick S, Wilden JD, Judd DB (2004) Direct synthesis of sulfonamides and activated sulfonate esters from sulfonic acids. J Am Chem Soc 126:1024–1025. doi:10.1021/ja0397658
Marcotullio MC, Campagna V, Sternativo S, Costantino F, Curini M (2006) A new, simple synthesis of \(N\)-tosyl pyrrolidines and piperidines. Synthesis 16:2760–2766. doi: 10.1055/s-2006-942488
Chen J, Dang L, Li Q, Ye Y, Fu S, Zeng W (2012) \(\text{ TiCl }_{4}\)-mediated direct N-alkylation of sulfonamides with inactive ethers. Synlett 4:595–600. doi: 10.1055/s-0031-1290332
Pelletier G, Powell DA (2006) Copper-catalyzed amidation of allylic and benzylic CH bonds. Org Lett 8:6031–6034. doi:10.1021/ol062514u
Bhuyan R, Nicholas KM (2007) Efficient copper-catalyzed benzylic amidation with anhydrous chloramine-T. Org Lett 9:3957–3959. doi:10.1021/ol701544z
García Ruano JL, Parra A, Marzo L, Yuste F, Mastranzo VM (2011) One-pot synthesis of sulfonamides from methyl sulfinates using ultrasound. Tetrahedron 67:2905–2910. doi:10.1016/j.tet.2011.02.060
Fukuyama T, Jow CK, Cheung M (1995) 2- and 4-Nitrobenzenesulfonamides: exceptionally versatile means for preparation of secondary amines and protection of amines. Tetrahedron Lett 36:6373–6374. doi:10.1016/0040-4039(95)01316-A
Kan T, Fukuyama T (2004) NS strategies: a highly versatile synthetic method for amines. Chem Commun 4:353–359. doi:10.1039/B311203A
Kumara Swamy KC, Bhuvan Kumar NN, Balaraman E, Pavan Kumar KVP (2009) Mitsunobu and related reactions: advances and applications. Chem Rev 109:2551–2651. doi:10.1021/cr800278z
Henry JR, Marcin LR, McIntosh MC, Scola PM, Harris GD Jr, Weinreb SM (1989) Mitsunobu reactions of N-alkyl and N-acyl sulfonamides: an efficient route to protected amines. Tetrahedron Lett 30:5709–5712. doi:10.1016/S0040-4039(00)76177-6
Shi F, Tse MK, Cui X, Gordes D, Michalik D, Thurow K, Deng Y, Beller M (2009) Copper-catalyzed alkylation of sulfonamides with alcohols. Angew Chem Int Ed 48:5912–5915. doi:10.1002/anie.200901510
Hamid MHSA, Allen CL, Lamb GW, Maxwell AC, Maytum HC, Watson AJA, Williams JMJ (2009) Ruthenium-catalyzed N-alkylation of amines and sulfonamides using borrowing hydrogen methodology. J Am Chem Soc 131:1766–1774. doi:10.1021/ja807323a
Cui X, Shi F, Tse MK, Gördes D, Thurow K, Beller M, Deng Y (2009) Copper-catalyzed N-alkylation of sulfonamides with benzylic alcohols: catalysis and mechanistic studies. Adv Synth Catal 351:2949–2958. doi:10.1002/adsc.200900490
Xu CP, Xia ZH, Zhuo BQ, Bwang YH, Huang PQ (2010) Efficient and chemoselective alkylation of amines/amino acids using alcohols as alkylating reagents under mild conditions. Chem Commun 46:7834–7836. doi:10.1039/C0CC01487G
Zhu M, Fujita K, Yamaguchi R (2010) Ruthenium-catalyzed tertiary amine formation from nitroarenes and alcohols. Org Lett 12:1336–1339. doi:10.1021/ol1002434
Cui X, Shi F, Zhang Y, Deng Y (2010) Fe(II)-catalyzed N-alkylation of sulfonamides with benzylic alcohols. Tetrahedron Lett 51:2048–2051. doi:10.1016/j.tetlet.2010.02.056
Terrasson V, Marque S, Georgy M, Campagn JM, Prim D (2006) Lewis acid-catalyzed direct amination of benzhydryl alcohols. Adv Synth Catal 348:2063–2067. doi:10.1002/adsc.200600236
Qin H, Yamagiwa N, Matsunaga S, Shibasaki M (2007) Bismuth-catalyzed direct substitution of the hydroxy group in alcohols with sulfonamides, carbamates, and carboxamides. Angew Chem Int Ed 46:409–413. doi:10.1002/anie.200602909
Wang GW, Shen YB, Wu XL (2008) Phosphotungstic acid catalyzed amidation of alcohols. Eur J Org Chem 2008:4367–4371. doi:10.1002/ejoc.200800413
Jana U, Maiti S, Biswas S (2008) An efficient \(\text{ FeCl }_{3}\)-catalyzed amidation reaction of secondary benzylic and allylic alcohols with carboxamides or p-toluenesulfonamide. Tetrahedron Lett 49:858–862. doi: 10.1016/j.tetlet.2007.11.176
Larock RC (1999) Comprehensive organic transformations, 2nd edn. Wiley, New York
Appel R (1975) Tertiary phosphane/tetrachloromethane, a versatile reagent for chlorination, dehydration, and P-N linkage. Angew Chem Int Ed Engl 14:801–811. doi:10.1002/anie.197508011
Soltani Rad MN, Khalafi-Nezhad A, Behrouz S, Asrari Z, Behrouz M, Amini Z (2009) One-pot synthesis of N-alkyl purine, pyrimidine and azole derivatives from alcohols using \(\text{ Ph }_{3}\text{ P/CCl }_{4}\): a rapid route to carboacyclic nucleoside synthesis. Synthesis 18:3067–3076. doi: 10.1055/s-0029-1216887
Soltani Rad MN, Khalafi-Nezhad A, Karimitabar F, Behrouz S (2010) An efficient one-pot synthesis of oxime ethers from alcohols using triphenylphosphine/carbon tetrachloride. Synthesis 10:1724–1730. doi:10.1055/s-0029-1218711
Soltani Rad MN, Khalafi-Nezhad A, Asrari Z, Behrouz S, Amini Z, Behrouz M (2009) One-pot synthesis of sulfonamides from primary and secondary amine derived sulfonate salts using cyanuric chloride. Synthesis 23:3983–3988. doi:10.1055/s-0029-1217020
Soltani Rad MN, Khalafi-Nezhad A, Asrari Z, Behrouz S (2010) Highly efficient one-pot synthesis of \(N\)-acylsulfonamides using cyanuric chloride at room temperature. Synthesis 15:2599–2603. doi: 10.1055/s-0029-1218819
Acknowledgments
The authors wish to thank Shiraz University of Technology research council for partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rad, M.N.S., Behrouz, S. \(\hbox {Ph}_{3}\hbox {P/CCl}_{4}\) as a highly efficient reagent for one-pot \(N\)-alkylation of sulfonamides from alcohols: a rapid route to \(N\)-alkyl sulfonamides synthesis. Mol Divers 17, 745–752 (2013). https://doi.org/10.1007/s11030-013-9471-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-013-9471-9