Abstract
The debate over how to best guide HIV-infected mothers in resource-poor settings on infant feeding is more than two decades old. Globally, breastfeeding is responsible for approximately 300,000 HIV infections per year, while at the same time, UNICEF estimates that not breastfeeding (formula feeding with contaminated water) is responsible for 1.5 million child deaths per year. The largest burden of these infections and deaths occur in Sub-Saharan Africa. Using this region as an example of the burden faced more generally in other resource-poor settings, we contrast the evolution of the clinical standard of care for infant feeding with HIV-infected mothers in high-income countries to the current international clinical guidelines for HIV-infected mothers and infant feeding in resource-poor settings. While the international guidelines of exclusive breastfeeding for a 6-month period seem to offer the least-worst strategy for reducing mother-to-child transmission of HIV during infancy while conferring some immunity through breastfeeding post-6 months, we argue that the impact of the policy on mothers and healthcare workers on the ground is not well understood. The harm reduction approach on the level of health policy translates into a complicated, painful moral dilemma for HIV-positive mothers and those offering them guidance on infant feeding. We argue that the underlying socio-economic disparities that continue to fuel the need for a harm reduction policy on infant feeding and the harm to women and children justify: (1) that higher priority be given to solving the infant feeding dilemma with improved data on safe feeding alternatives, and (2) support of innovative, community-driven solutions that address the particular economic and cultural challenges that continue to result in HIV-transmission to children within these communities.
Similar content being viewed by others
Introduction
The debate over how to best guide HIV-infected mothers in resource-poor settings on infant feeding is more than two decades old. During that time, clinical guidelines on the best approach for mothers in developed countries settled relatively quickly on aggressive anti-retroviral treatment for the mother to prevent mother-to-child transmission of HIV, followed by formula-feeding for the infant, bringing the risk of HIV-transmission to the infant down to less than 2% [1–3]. Clinical guidelines for mothers in developing countries, on the other hand, have oscillated confusingly between formula-feeding, an informed choice between formula and breastfeeding, and exclusive breastfeeding (EBF), settling most recently on a policy of exclusive breastfeeding for a period of 6 months [4]. Well-meaning researchers, aid workers, local healthcare workers and midwives have struggled to advise women in the face of horrible circumstances: for those women living in severe poverty, the lack of clean water can make powdered formula-feeding deadly, and widespread malnutrition means that breastfeeding may be an infant’s best chance to receive the nutrients and antibodies needed to survive in an environment where food is scarce and infectious diseases are widespread. In these circumstances where gender inequalities are often pervasive, the women must also confront stigma associated with activities that reveal a positive HIV diagnosis. This dire situation for the mothers and those advising them can be characterized as a moral dilemma in the true sense. No matter what the mother does (and no matter what we advise the mother to do), the infant is subject to potentially grave harm.
The implicit ethical reasoning behind the clinical guidelines and among those doing this difficult work is that the appropriate though regrettable response to such dilemmas is to act to balance the maternal and infant benefits and to minimize the harm to both (see [5]). While this is a reasonable moral calculus for crafting health policy in the face of seemingly intractable trade-offs, there is a risk of overlooking the human toll such a policy exacts when implemented in the field. Missing from discussion of the current guidelines is a frank acknowledgement of what lies beneath the infant feeding dilemma. By that, we mean two things: We are missing an appreciation for the impact of the harm reduction approach on individual women, a contextual sense of how real women struggle with nourishing their young while dealing with all of the concomitant challenges of living with HIV. We are also missing a frank recognition of the broader context of the feeding dilemma and the social, health, and economic disparities behind it. Here, we hope to address these issues more directly as a way of further motivating the urgency of the problem, and the desperate need for a better solution. A better solution, as we argue here, will not only address the immediate predicament of HIV-infected mothers living in poverty, but also the underlying disparities that give rise to the predicament. For those not familiar with the public health data on infant feeding and HIV or the debate over international policy for resource-poor settings, we begin with an overview of current data and evolving policy on the issue. We then turn to a detailed discussion of how the feeding dilemma is experienced by women and health care workers in the field, and offer an ethical analysis and recommendations for improving options for women in low-income settings. Our primary aim is to move infant feeding up on the global agenda, with greater attention to creative alternatives for safe infant feeding practices for women living in poverty.
The evolution of clinical guidelines on HIV-infected mothers and breastfeeding: the view from above
The battle against the HIV/AIDS pandemic continues on several important fronts: preventing new cases, improving quality of life for those living with the disease, and finding support for the millions of children orphaned by the disease. One issue that cuts across all three fronts is the predicament faced by pregnant women who are infected with HIV and living in extreme poverty, a disproportionate number of whom live in Sub-Saharan Africa. According to the UNAIDS global data on HIV/AIDS, an estimated 13.3 million women (>15 yrs.) in Sub-Saharan Africa were living with HIV as of 2006. The same data estimate that children under 15 account for one in every seven new HIV infections and one in every six AIDS-related deaths [6]. In Sub-Saharan Africa, HIV/AIDS contributes to roughly 8% of all childhood deaths, and in those areas where the prevalence of HIV among pregnant women is higher than 35%, HIV/AIDS contributes to as much as 42% of childhood mortality [4]. The majority of cases of HIV-transmission in children continue to be through mother-to-child transmission (MTCT), with breastfeeding accounting for as many as 40% of new infections (see Table 1) [6]. HIV transmission from mother-to-child can be substantially reduced by administering antiretroviral therapy (i.e., zidovudine [ZDV], ZDV plus lamivudine (3TC) and nevirapine [7]) to the mother during pregnancy, labor and delivery, and then to the newborn. Therefore, a significant focus of intervention efforts in Sub-Saharan Africa is on identification and enrollment of HIV positive pregnant women into PMTCT programs. In South Africa, for example, PMTCT enrollment in 2006 was 186,646 (72.7%) and for the period January to September 2007 was 144,506 (56.2%) [8]. Even if medications are commenced during labor and delivery, the rate of perinatal transmission can still be decreased to less than 10% [9]. For women with viral loads greater than 1,000 copies/ml, elective cesarean sections are recommended as they can reduce the rate of MTCT to 2% or less [10]. Unfortunately, cesarean surgery is not typically available or safe for women in resource-poor countries. Earlier in the HIV pandemic, Bulterys et al. concluded that in developing countries, HIV-positive women who had cesarean deliveries had a much higher mortality rate in comparison to women who had undergone vaginal deliveries [11]. Because of such evidence, vaginal deliveries have been recommended for women in resource-poor settings. To further optimize the reduction of perinatal transmission, replacement feeding is recommended as an HIV post-natal preventative measure [4].Footnote 1
Most recently, a series of studies in Botswana, Malawi, Ethiopia, Uganda, Tanzania and India demonstrate that antiretroviral treatment given postnatally to an HIV-infected mother and/or as prophylaxis to the uninfected infant can reduce the rate of transmission from mother to infant through breastfeeding [12, 13]. These studies represent the first attempt to determine the potential impact of administering antiretroviral drugs postnatally to mothers or infants. While there is excitement about the potentially positive impact of decreasing MTCT through breastfeeding, important questions remain regarding the safety, efficacy, and sustainability of the approach, including optimal duration of prophylaxis, the long-term effects on the infant, the long-term impact on maternal health (particularly the effects of interrupting the mother’s treatment regimen among those mothers on HAART), and concerns about increasing the prevalence of drug resistant strains [14, 15]. Of greatest concern in the context of Sub-Saharan Africa is the question of sustainability. While international organizations, national governments, and activists have been instrumental in addressing the cost of ARVs and facilitating distribution [16, 17], significant barriers remain in the lack of skilled clinical infrastructure to deliver drugs, conduct tests, and monitor patients’ disease progression. It is as yet unclear how this data ought to impact feeding policy.
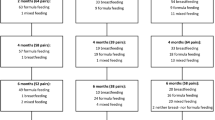
Since the first reported case of AIDS in 1981 [18] and the first recorded case of mother-to-child transmission via breastfeeding in 1985 [19], policy makers have been pressured to make recommendations on infant feeding for HIV-infected mothers, often with little or incomplete empirical evidence. While data have supported a stable standard of care for women in developed countries (even those women living in poverty in developed countries), decisions for HIV-infected mothers living in developing countries have presented the greatest challenges, as reflected through fluctuating policy (see Table 2). The infant feeding debate persists because on one hand, breastfeeding is thought to be responsible for about 300,000 HIV infections per year while UNICEF estimates that not breastfeeding (typically, formula feeding with a contaminated water supply) is responsible for 1.5 million child deaths per year [20]. UNAIDS reports that more than 90% of the estimated 640,000 annual infections occurring in Sub-Saharan Africa occur through mother-to-child (perinatal) transmission. Of such infections, it has been estimated that between 30 and 45% of perinatal HIV transmissions occur through breastfeeding [6, 21, 22].
HIV transmission via breastfeeding is pervasive in places where breastfeeding is the predominant form of infant feeding as a result of economic conditions and cultural norms. Investigators observe in “settings where breastfeeding is widely practiced and usually prolonged 1 year after birth, the overall risk of HIV transmission through breast milk was estimated to be 8.9 new cases per 100 child-years of breastfeeding” [23]. In light of the infant feeding dilemma, a number of research studies have aimed to examine safety and efficacy of infant feeding alternatives to prevent post-natal HIV transmission through breastfeeding. Several studies have highlighted the need to develop strategies to reduce childhood illness such as diarrheal and respiratory illnesses in developing countries. These studies support the view that “breastfeeding by HIV-infected women is safer under the worst conditions in resource-poor countries” [24, 25]. The first ever randomized control trial conducted recently among Botswanan women to compare formula feeding to breastfeeding with zidovudine prophylaxis reported that although their study did not lend definitive support to the use of infant zidovudine prophylaxis to prevent HIV-transmission via breastfeeding, their study instead revealed relatively high risk of early infant mortality associated with formula feeding [21].
Research conducted by Coutsoudis and her research team in 1999 first established that for HIV-positive mothers, exclusive breastfeeding—with no other oral food or drink to the baby, not even water—as the most biologically advantageous and least harmful to the infant [26, 27]. Coutsoudis et al. stated “exclusive breastfeeding may offer HIV-1 infected women in developing countries an affordable, culturally acceptable, and effective means of reducing mother to child transmission of HIV-1” [27–29]. Unfortunately, mixed breastfeeding—introducing other foods or liquids along with intermittent breastfeeding—is the most common feeding alternative in South Africa [20]. For this reason, among others, the WHO, UNICEF, and UNAIDS did not initially accept these findings as sufficient evidence to amend policy. However, in October 2000, WHO, UNICEF, and UNAIDS clarified the guidelines by recommending exclusive breastfeeding for HIV negative mothers and HIV positive mothers who could not afford to safely sustain formula feeding. The most recent joint guidelines state the following:
When replacement feeding is acceptable, feasible, affordable, sustainable and safe, avoidance of all breastfeeding by HIV-infected mothers is recommended; otherwise exclusive breastfeeding is recommended during the first 6 months of life.Footnote 2
But while coming down firmly on the side of formula feeding under ideal conditions, the WHO also recognized that those conditions are nearly impossible to meet in Sub-Saharan Africa [31]. The harsh reality is that replacement feeding is hardly ever “acceptable, feasible, affordable, sustainable, and safe.” The WHO coordinator for infant and neonatal health, Dr. Jose Martines, stated in telephone interview that the recommendation for HIV-infected mothers to exclusively breastfeed was based on research that demonstrated the risk of other infections was significantly lower from exclusively breastfeeding. He added, “it is an area where public policy decisions are made with less information than one would hope to have but we don’t want to be so slow when there is an opportunity to save lives” [31].
The implicit reasoning behind the WHO guidelines for infant feeding among HIV-infected women in resource-poor settings is consequentialist reasoning, more specifically, cost-benefit reasoning. Based on the data available, in developing regions such as Sub-Saharan Africa, there are more infant deaths caused by formula feeding with contaminated water (and possibly by a concomitant decrease in immunity in those infants who did not receive antibodies from their mothers’ breast milk) than there are infant deaths caused by HIV-1 transmission. Further, compared to exclusive breastfeeding, mixed feeding methods increase the rate of HIV transmission from mother to child, perhaps due to an inflammatory reaction in the gut caused by early introduction of insoluble substances. The least-worst option in the face of the current data is to encourage women to breast feed exclusively for the first 6 months of an infant’s life, then switch to formula feeding and other foods and liquids. Within the constraints of severe scarcity and poverty, minimizing the harm done to both mother and child is the most reasonable, although deeply regrettable, course of action. The question remains: how we should view and rectify the overt disparities inherent in the joint global guidelines? The first step is to frankly recognize the complex socio-economic disparities underlying the compromise approach to clinical guidelines and to remind ourselves that least-worst, harm reduction approaches should be viewed as interim, stop-gap measures, and not final solutions. Moreover, we hope that focusing on the contextual challenges of the infant feeding dilemma will highlight the importance of social scientists, medical scientists, policy makers, health care providers, and community members collaborating to find solutions that would obviate the need for such hard choices.
The infant feeding dilemma: the view from below
While the “view from above”—a look at the stark differences in standard of care between contexts—is an important means of illustrating the moral disparities inherent in the feeding dilemma, it is an incomplete picture of the dilemma. The data we presented in the last section regarding rates of transmission, infection, and mortality are “data from above,” and they are crucial for understanding the enormous scope of the problem. At the same time, it is important to remember the ethical significance of particular lives. Although they appear as numbers when viewed from above, each represents a life lived, or lost, and all the struggles in between. To make the case for increased research to find better solutions to the infant feeding problem, we need a better understanding of the contextual challenges faced by women in these communities. Here, we take Paul Farmer’s lead in considering “the view from below” as we examine the ethical issues faced by aid workers, midwives, and HIV-infected mothers in their daily activities and communities [32]. We will focus on a representative case of an HIV-infected mother visiting a clinic in South Africa. Our aim in the case and literature analyses is twofold: The problems encountered at the community level, taken together, are often cited as insurmountable barriers to achieving a more equitable global feeding policy. Any successful move toward a more equitable policy must then address these barriers. More generally, it is also important to understand the types of problems that fuel health disparities like that seen in the infant feeding dilemma. This, again, is an attempt to see beyond the dilemma itself to its root causes. We begin with a real case to help illustrate the central issues at stake for HIV-positive women with newborns, particularly, in low-income areas of Sub-Saharan Africa.
Representative clinical scenario: South Africa Footnote 3
The patient, Ms. N. is a 25-year-old woman infected with HIV from Kwazulu-Natal who came to the clinic for the first time at 8 days post delivery. She was enrolled in a care and treatment program at the University and was classified as being in Stage-3 of her illness, with oral thrush; however, she had never received prevention of mother-to-child transmission therapy (PMTCT). She was admitted for gastroenteritis once during her pregnancy. She had tuberculosis in 2002 and finished the course of medication. At the time of enrollment, her CD 4 count was 134 cells/µl and her viral load 140,000 copies/ml. She delivered around her 36th week of gestation. When Baby N. was born, he was enrolled on the same day as mom at 8 days of age. Mom is unemployed and single with three children. She is receiving a Child Support Grant (CSG) for her two other kids. She was told at the hospital where she delivered that she must not breastfeed as she is HIV infected. No one asked her about her socio-economic conditions or provided her with any counseling surrounding infant feeding choices. She lives in a two-room Reconstruction and Development Programme (RDP) house with thirteen other people. She has no electricity, but has piped water. She has disclosed her HIV status only to her mother. Ms. N was struggling to make up the formula feeds as she could not afford the paraffin to boil the bottles to sterilize them and to boil water for the feeds (channeling assistance funding to feeding her extended family members). The baby suffered from severe diarrhea. Ms. N was then told by her physician to breast feed because it was thought to be best for the clinical improvement of her child especially for the oral thrush the infant persistently experienced.
At 4 months, the baby’s DNA polymerase chain reaction (PCR) came back positive with a CD 4 of 23% (918). On enrollment the baby was assessed as asymptomatic and he came back 12 days later with oral thrush and dermatitis for which he was treated. He has continued to suffer several bouts of severe diarrhea. Once the baby had been tested and found to be HIV-positive, the counselors tried to help the mother to begin lactating but they were not successful because such a long time had elapsed. The mother was very distressed by her baby’s HIV-positive diagnosis and the management of her care as well as that of her infant.
This case illustrates the very difficult situation faced by many HIV-infected mothers in the world regarding management of pregnancy, management of illness, postnatal management of babies’ nutrition and immunity, and concerns about stigma and social support. At first glance, the problems may seem overwhelming, especially to the woman and health care workers attempting to make sense of the morass. Still, it is important to ask from an ethical point of view, do these complex barriers justify the long-term acceptance of the current feeding policy for women who are pregnant and living with HIV and in poverty?
Formula feeding among HIV-positive mothers in high and middle-income settings has become so standard that some hospitals would consider the scenario of an HIV-infected mother who disregards her physician’s advice to formula feed and instead breastfeeds her infant to be grounds for calling Child Protective Services to prevent harm to the baby [33, 34]. And yet millions of HIV-positive women in impoverished parts of the world are told, albeit with regret, to choose the lesser of two harmful options and breastfeed exclusively. This places the central moral disparity in the infant feeding debate in the global context: What warrants recommending a course of action that would otherwise be considered child neglect, and possibly assault, in privileged parts of the world? Does the scarcity of resources, minimal access to health care, and various cultural practices favoring breastfeeding justify the asymmetry of practice? What we wish to emphasize here is the importance of noting the deep moral disparity in the standard of care for an HIV-infected mother in a developed country, and the situation of millions of mothers living in extreme poverty in developing countries.
Two types of barriers are typically cited as a justification for the asymmetry in clinical guidelines for breastfeeding among HIV-infected women in resource-poor settings. First are the substantial economic barriers posed by severe poverty: a general scarcity of resources, lack of food security, lack of adequate (if any) access to HIV treatment and general health care, resulting malnutrition, other infectious diseases and childhood illnesses, and environmental pollution of water sources. We will characterize this class of difficult problems as economic-environmental barriers. The second class of barriers cited as a reason for the disparity in feeding guidelines includes social and cultural barriers. This class includes serious gender inequalities, stigma and domestic violence associated with an HIV diagnosis, lack of HIV serostatus awareness, lack of education among women and health care workers, and cultural practices that support mixed breastfeeding [35, 36]. We will consider each of these more closely with an eye to identifying programs that will successfully overcome these barriers.
Economic and environmental barriers to safe alternative feeding methods
The most entrenched barriers to a program of alternatives to breastfeeding for HIV-infected women are the familiar barriers raised by severe poverty. These conditions fuel the current clinical guidelines, endorsed by WHO, UNAIDS, and UNICEF, to promote exclusive breastfeeding when alternatives are not “acceptable, feasible, affordable, sustainable, and safe.” In the worst conditions in resource-poor countries, calculations show breastfeeding by HIV-infected women is safer than the alternatives [24, 25]. We have already mentioned the most standard alternative, endorsed in developed countries, of formula feeding. Again, with rates of water contamination in impoverished villages and urban neighborhoods, coupled with an infant’s compromised immune system due to baseline malnutrition and lost immunity from not breastfeeding, the pediatric loss of life due to dysentery, cholera, other water-borne infections, and childhood illnesses is higher than the mortality rate for HIV-infection [5].
There have been a number of creative attempts to come up with alternatives to both breastfeeding and formula feeding in impoverished settings (see Table 3). Among these alternatives are the use of modified animal milk, heat treatment of breast milk, wet nursing, and donor breast milk. As one can see by the table, there are complicated cost-benefit trade-offs associated with each option, fueled by many of the same economic barriers that keep formula feeding from being a safe and viable alternative. If we can better identify the sources of risk, we can work towards ascertaining best practices for feeding infants born to HIV-infected mothers in places of high HIV burden and limited resources.
Social and cultural barriers to safe alternatives: gender inequalities and stigma
Cultural norms and serious gender inequalities often compound the economic barriers to implementing infant feeding programs in severely impoverished communities. In our search for safe feeding alternatives, it is important to understand and take these norms into account when proposing new practices within communities. As we see in the South African case above, women in developing countries, particularly in Sub-Saharan Africa, are often faced with a confusing array of feeding options and conflicting advice regarding the best way to feed their babies while, at the same time, avoiding postnatal mother-to-child HIV transmission through breastfeeding. Sadly, the deeper inequalities affecting many women in such settings mean they must not only navigate among confusing options, but also lack the clear support, education, and voice in the decisions that affect their lives and their infants’ lives. Further, because breastfeeding is the norm, any use of an alternative method can signal HIV-positive status to the rest of the women’s community, and so they must confront the stigma often accompanying their diagnosis.
Among the alternatives considered above (see, again, Table 3), economic barriers aside, each of these alternatives poses risks to the mother associated with disclosure of her HIV-status. The process of heat treatment, for example, would be very difficult to conceal from others, certainly from extended family members and others in one’s village or neighborhood [37]. If the woman is observed, she may be ostracized by family members or members of her community. The same stigma often accompanies bottle-feeding and cup feeding and wet nursing in some communities. To better understand this from the woman’s point of view, consider the difficulty of a daily or weekly activity, such as riding a bus. Women traveling on long journeys by public transport encounter challenges when others notice their crying infants. Generally, fellow passengers will encourage women to breastfeed their babies in an effort to quiet them on the bus. The woman may eventually give in to the social pressure and breastfeed her baby even if she knows that she may risk transmitting HIV to her baby because of her condition. On the other hand, she could choose to endure a long bus ride consisting of an upset baby and frustrated, judgmental passengers. It is important to acknowledge the role of social acceptance in every day social and parenting activities.
In a study conducted among a sub-sample of forty HIV-infected women in South Africa by Doherty and the research team, women reported low levels of self-efficacy and decision-making capacity related to infant feeding which prevents them from carrying out certain feeding practices including exclusive breastfeeding [38]. One participant commented:
When they see me coming with tins they laugh at me. They say I have HIV, I tell them I do not have AIDS, it is because of TB and a lot of people know I have TB and I hide the tins (mother aged 22 years, infant aged 8 months, formula fed) [38].
Stigma can also manifest itself within the folds of the family. The following scenario from Seidel et al. illustrates the family’s influence on a woman’s infant feeding decision:
Happiness had read up about HIV on her own initiative. She knew the risks and had decided not to breastfeed. She had managed not to breastfeed. She had managed to disclose to her husband, but not to extended family. She had prepared bottles and hid these in the washroom. In the ward, she would surreptitiously bottle-feed the baby adopting the position of breastfeeding from under blankets. One day she was caught and an explanation was demanded as to why she was not breastfeeding. In order to explain what was seen as disobedient behavior she felt obliged to reveal her HIV status and expressed concern about this outing. After that she was allowed to bottle-feed [35].
The woman in this case was permitted to breastfeed after disclosing her status; however, all women are not as fortunate and might instead revert to breastfeeding to avoid questioning and other potential negative consequences.
The everyday dilemmas revealed by these women’s stories are not fueled simply by the lack of access to infant formula or a shortage of clean water; the economic issues are one part of a much deeper social problem. Certain behaviors and images—a mother carrying tins of formula from a clinic, or a crying baby being bottle-fed—are now widely symbolic of HIV infection. The stigma associated with such daily but symbolic behavior may deter a woman from using formula, even when it is economically feasible and affordable. In Sub-Saharan Africa, where accessibility to HIV treatment continues to be limited, there is a greater tendency toward blame, shame, stigma and fear embedded in society. In a place where HIV is highly stigmatized and where breastfeeding is the cultural norm, feeding alternatives such as heat treatment of breast milk, exclusive breastfeeding, wet nursing, donor breast milk and formula feeding have been deemed unfeasible, unacceptable and unsafe, not only because of economic barriers, but because of the repercussions to the mother and infant if the mother’s HIV serostatus were to become known. Doherty and research team found that the women’s fear of having one’s status revealed and the fear of stigma “weakened the ability of mothers to resist entrenched family and community norms that encourage early introduction of fluids and foods and that question non-breastfeeding” [38]. Such concerns mirror problems faced in the general adult population, involving, for example, skipping a dose of prescribed medicine when in the presence of other people or refusing to stand in line for HAART for fear of being seen by others [39]. Indeed, since the beginning of the HIV epidemic, people living with HIV/AIDS (PLWHA) and their respective social groups, globally have been subjected to stigma [40]. In seeking solutions, anti-stigma and sensitization programs need to be an integral element. This has been well recognized in the HIV-prevention and treatment programs for adults and should be expanded in a more targeted fashion to infant feeding programs.
Women in many of these communities also face more systemic challenges of gender inequality. From the time that they are young girls, they are often overlooked when it comes to scarce educational opportunities, decision-making, and information about their health. HIV-infected women living in poverty face a lack of general health care, social support, and education regarding their disease and best practices for infant feeding. It is not surprising, then, that many HIV-infected women continue to breastfeed because they are either unaware that they are HIV-infected or they are uninformed that breast milk can transmit HIV. Moreover, women who know their serostatus often have good reason to keep their status from their male partners, given very real concerns about abandonment or abuse [35, 37, 41]. An extensive literature review reveals that studies support the claim that pregnant women are even more reluctant to disclose their status during such a vulnerable time for fear of abuse and abandonment [42].
Women also receive confusing and sometimes contradictory messages from groups with competing clinical aims, and sometimes competing political agendas. Coutsoudis maintains that “the HIV pandemic has introduced dilemmas for health policy makers and health care workers, and has resulted in a polarization between those whose mandate is preventing the spread of HIV (and therefore would see the importance of replacing breastfeeding) and those whose mandate is child survival and therefore promote breastfeeding as one of the pillars of child survival” [28]. Similarly, as we have seen with the controversy surrounding the Nestle formula boycott, politics can often drive recommendations or fuel fears and mistrust about the recommendations being offered [37]. When a corporation has a vested interest in promoting the sales of powdered formula, or an organization has strong ideological views about the cultural value of breastfeeding, it is important for women to be empowered to recognize these conflicts of interest when considering the advice offered by organizations. With groups such as La Leche League or corporations such as Nestle, it may be difficult for a woman living in poverty without an education, literacy, access to news, or an active voice in her community, to sort out the possible political motivations behind otherwise well-meaning programs and guidelines. This illustrates the need for more women-centered programs that attempt to elicit views, beliefs, and preferences from the women themselves while giving them the skills to network and educate themselves about their choices and options.
Both the economic and cultural barriers considered here are often presented in the literature as being largely insurmountable barriers to improving the lives of HIV-infected women and the health of infants born to HIV-infected mothers. In response to this skeptical stance, we have described some of the difficulties in detail to help us better understand the infant feeding dilemma as viewed by individual women living in severe poverty without adequate economic and health resources or social support to nourish their infants in the safest way possible. We also hypothesize that this skeptical stance has allowed the disparity in feeding practices to persist. That women since 1987 in developed countries have been able to seek safe alternatives for infant feeding and, nearing 2009, women in developing countries are still being advised, regrettably, to breast feed and risk HIV transmission to their infants as a “least worst option,” indicates that we need to think more creatively about solving these problems. The most recent maternal and infant antiretroviral prophylaxis studies summarized briefly in section one may represent an interim solution to the deeper problem, but should not be considered a substitute for addressing the underlying causes of the feeding dilemma. In addition to the serious concerns mentioned regarding safety, efficacy, and sustainability, the maternal or infant prophylactic studies have not addressed the way in which the inconclusive data, changing guidelines, and social and cultural factors confound the already difficult choice facing HIV-infected mothers and those offering them guidance and support. If the relative risk of postnatal infection via breast milk can be reduced by 20% at age 6 months (6.9% as compared to 9.0%), as reported in the Six-Week Extended Nevirapine (SWEN) study [43], the mothers in the study arm are still faced with the choice of exposing their infants to a 6.9% chance of contracting HIV with unknown implications for long-term morbidity associated with the drug regimen or with early weaning. Prophylaxis can reduce postnatal transmission but the only certain way to avoid transmission is not to breast-feed. In settings where replacement feeding also carries sufficient risk due to water conditions, risks of infectious diseases, and high prevalence of stigma associated with replacement feeding, the feeding dilemma has not been solved. What is still missing from these studies and from the infant feeding debate is due consideration of the voices of the women faced with the feeding dilemma as it persists, even in light of the most recent developments in prophylactic treatment of mothers and infants.
A call for creative, integrated solutions
It is crucial not to downplay the formidable and often overwhelming nature of the barriers to a better feeding solution for HIV-infected women living in dire poverty. However, one can take these barriers very seriously, and not be defeated by them. What we wish to challenge here is the idea that the social and economic barriers to more equitable global feeding guidelines justify the moral asymmetry and injustice in the divergence between the developed and developing countries. Beginning with the problem of stigma, we will canvass some promising solutions to the barriers discussed above. To address the barriers described in the previous section, any successful overall solution will include three integrated components: (1) aggressive prevention and treatment programs, (2) aggressive anti-stigma campaigns, and (3) better coordination with those working on economic and infrastructure problems, such as clean water programs. We turn now to some constructive approaches that integrate the community influence to improve infant feeding practices for HIV-infected mothers.
As a phenomenon, stigma has been identified as an obstacle to HIV prevention efforts, treatment, and care. As Ogden and Nyblade have put it, “Stigma is believed by many policy makers to be too cultural, too context specific and too sensitive to be addressed meaningfully” [44]. There are indeed stark differences in the pervasiveness of stigma between developed and developing countries. Such has been attributed to differences in access to treatment, HIV disclosure, education, public health campaigns, and the historical context from which stigma arises. Castro and Farmer have observed that AIDS-related stigma has decreased in Haiti where the availability of HIV testing, counseling, and HAART has increased [45, 46]. While we are sympathetic to the claim that AIDS-related stigma presents challenges for the uptake of HIV treatment, care, and prevention efforts, we strongly support the positions of others who view stigma (internalized and actualized) as a human rights violation and a representation of deeper systemic issues that we typically find difficult addressing further complicating the empirical measurement of stigma as a concept [45, 47]. Those who accept the claim that all supplemental forms of infant feeding for an HIV-infected mother will result in a stigmatizing disturbance as an impetus for inaction without considering the core determinants of stigma are acquiescing in and even perpetuating the infant feeding dilemma.
The social, cultural and political situation for many women in Sub-Saharan Africa simply exacerbates stigma. However, stigmatization should not be viewed as an insurmountable barrier, but rather as a reminder of the urgency for the development of more effective community-based public health programs. What lies beneath the infant feeding dilemma, in significant part, is a failure and/or reluctance to address gender inequalities, poverty, political violence as mechanisms of structural violence emphasized by Castro and Farmer [45]. Ndaba and Burns summarize the affect of stigmatization on women in Southern Africa:
The stigmatization of HIV-positive women, who bear and give birth, as “infectors,” and the power dynamics in women’s homes between infected mothers, their male partners, and their mothers-in-law and grandmothers, as well as male and female neighboring and kinsfolk, is recognized in much published commentary on the epidemic, but is still not well understood [48].
In Zimbabwe, the example of “the blue bag case” illustrates health professional’s laudable efforts to destigmatize unintentionally created stigma. Among persons seeking health care in a research clinic in Zimbabwe, those who were HIV-negative would receive a blue bag with condoms and educational materials; those who were HIV-positive would leave the clinic empty-handed. As a result, people in the community took note of those individuals who left the clinic without a blue bag, and they would then sometimes spread word about the individual’s HIV status within the community. The problem wasn’t ameliorated until a community member, who was HIV-positive and who feared being stigmatized, refused to leave the clinic without a blue bag. Health care workers then correctly recognized that certain individuals were marginalized by the clinic’s “blue bag” practice. As a consequence, everyone who visits this particular clinic now receives a blue bag regardless of his or her HIV serostatus.Footnote 4
Another instance of correcting unintended HIV-related stigma lies within the phrase “Prevention of Mother-to-Child Transmission.” Employing this phrase is now very much out-of-step with structural changes aimed to enhance a woman’s autonomy. Even though not yet universally adopted, the phrase Prevention of Parent-to-Child Transmission (PPCT)Footnote 5 is being promoted. Mother-to-child transmission implies to many that the mother is the primary or only source of infecting her baby with HIV, while her partner in most cases is equally responsible for the baby’s HIV-positive serostatus. Many women in South Africa are infected with HIV through monogamous relationships with their male partners. Using the PPCT terminology alleviates some of the blame and shame inflicted on the mother and reminds individuals that the mother could easily have been infected through voluntary and involuntary intercourse with a man, receiving contaminated blood from non-sterile instruments or from contaminated medical procedures, thus infecting her baby. It wisely reminds others that the mother is not the primary source of infectivity.
Both examples illustrate small-scale, practical, but creative responses to combat stigma. One strategy seeks to circumnavigate existing stigma. The other uses counter-symbolism and the power of chosen language to alter attitudes about HIV/AIDS. In Lusaka, Zambia, for example, where there has been an alarming increase in the number of rapes of young girls by HIV-infected men, a public health effort has installed billboards with the image of a very young girl and the words, “Sex with me will not cure HIV/AIDS.” These public attempts to reverse a widely-held conception are coupled with more aggressive attempts to empower young girls and boys with correct information about HIV transmission and children’s rights not to be harmed or sexually assaulted, as well as the establishment of rape support and anti-violence laws [49].
In an attempt to feed their infants, women infected with HIV have already begun implementing practical solutions to reconcile the “infant feeding dilemma.” Such is represented through mothers going to the clinic, collecting their milk and then tearing off the cover which displays the type of formula and generates questions from community members [38]. As with the blue bag case, this scenario should serve to convince researchers of the importance of putting community-based participatory research into practice. Those living with HIV have already found innovative ways to avoid public condemnation. Listening to the populations impacted by the epidemic and working with them to improve their quality of life by capitalizing on their employed environmental coping mechanisms is critical to designing efficacious programs and interventions.
An excellent example of the integrated, community-driven approach can be found in the work of biomedical scientist Dr. Anna Coutsoudis in South Africa. Beyond establishing the empirical evidence for the greater harms of mixed feeding practices over breastfeeding and alternatives, Dr. Coutsoudis has taken a multi-disciplinary approach to uncovering and overcoming the structural barriers to safer feeding practices for HIV-infected women in the region. By combining medical research, social science research, transdisciplinary international collaboration and community-based efforts with women’s groups, she has helped set up a number of promising alternative feeding programs. In Durban, for example, she is working with an American mother who developed a non-profit organization to provide donated milk to babies in South Africa. The Ithemba Lethu breast milk bank relies on a network of mothers in the city to provide nutritious and immunity-strengthening breast milk to AIDS orphans in Durban. They sought the advice of local blood and international breast milk banks on screening techniques for potential donors. For pasteurization, they rely on a donated industrial pasteurizer to heat treat the milk at a temperature that preserves the nutritive and immunity-protecting properties in the milk. Mother’s groups in the area have been involved in the development and the ongoing operation of the program, and UNICEF has contributed funding and technical support. As Dr. Coutsoudis along with other biomedical and social scientists fight for better ARV treatment and preventive programs, their efforts to work aggressively for safer interim solutions have saved some babies’ lives [50].
The socio-cultural and economic barriers surrounding infant feeding indicate the need to explore new paradigms to guide infant feeding interventions to assist HIV-infected women in Sub-Saharan Africa. A recent qualitative study conducted in Malawi by Bezner and research team found the multiple roles of grandmothers in agriculture, child feeding and social relations warrants their inclusion in interventions [51]. Moreover, previous research studies conducted among HIV-infected South African women indicate that male partners, brothers, health care workers, kinsfolk and other community members critically impact women’s infant feeding decisions [35]. Theoretical models must incorporate the mother, extended family and neighborhood to change infant feeding behavior. In the case of infant feeding, researchers assert the following regarding existing public health models:
The public health approach of teaching mothers new knowledge about child feeding practices inadequately addresses the power dynamics and ignores prevailing alternative explanations for child illness that exist within communities [51].
A compelling example of community involvement in an infant feeding public health initiative is the Breastfeeding, Antiretroviral Drug, and Nutrition (BAN) study. The BAN study is an un-blinded clinical trial in Lilongwe, Malawi, focusing on the safety and efficacy of antiretroviral and nutritional interventions to reduce mother-to-child transmission of HIV during breastfeeding [52]. This is a comparative clinical trial among HIV-infected women and their infants to determine: (1) the benefit of nutritional supplementation given to women during breastfeeding, (2) the benefit and safety of antiretroviral (ARV) medications given either to infants or to their mothers to prevent HIV transmission during breastfeeding and (3) the feasibility of exclusive breastfeeding followed by early, rapid breastfeeding cessation. One key feature of this study is the formative research involving Malawians in the design of the clinical trial protocol. To examine the feasibility of adhering to the WHO’s early cessation recommendation for HIV-infected mothers, researchers involved mothers of undisclosed HIV status in taste trials. Mothers living in food insecure households reported cultural and economic pressure to share the infant’s supplements among other family members and neighbors. Based on the formative research, peanut butter was selected for infants and “the supplement was named ‘Nutrition for Breastfeeding Mothers’ to minimize any stigma associated with its use in the context of the study and to possibly reduce sharing.” Investigators noted that families were additionally provided with a small bag of maize to reduce sharing of the nutritional supplement. By soliciting the voices of mothers, extended family and community members, the BAN investigative team addressed socio-cultural and economic factors that complicate infant feeding for HIV-infected Sub-Saharan African mothers [52]. Albeit more challenging, changing behaviors that are deep-seated in culture, tradition and social norms can be accomplished through educational efforts and through the application of community-based participatory models [53, 54].
At the same time, it is important to appreciate the significant challenge of incorporating evolving data that may vary across regions and socio-economic settings, into a clear and consistent message in feeding policy that can be implemented in practice and yet is also sufficiently flexible to adapt to new findings. A recent commentary published by Coovadia and Coutsoudis highlighted the danger of HIV-infected Sub-Saharan African mothers avoiding breastfeeding completely or stopping at 6 months, consistent with the WHO guidelines [55]. Recent data from studies conducted in African countries (Malawi, Zambia, Kenya, Uganda, Bostwana, and Cote d’ Ivoire) demonstrate high rates of infant mortality, most often resulting from dysentery-associated illnesses from such avoidance or early cessation of breastfeeding based on global health recommendations. Coovadia and Coutsoudis stressed the urgency to incorporate the implications of data into new or revised global feeding policy. The team recommends: “For the overwhelming majority of women in developing countries: make breastfeeding safe by minimizing HIV transmission and maximizing health and survival in infants and children. For a minority of women in developing countries: make formula feeding safe by minimizing morbidity and mortality and maximizing health and survival for infants and children” [55]. They additionally challenged researchers to explore new ways of making breastfeeding safer for HIV-infected women as well as ramping up evidence-based interventions for HIV infected women. Recognizing the inequalities associated with the contaminated water supply in many developing areas of the world, the study team emphasized the need to make formula feeding safer for infants as well. A successful feeding policy will in part be generated from the ground up, allowing a quicker response in practice guidelines to epidemiological findings in the field.
Conclusion
To return once again to the case with which we began our discussion, we wish to emphasize the injustice that is masked when the moral decision facing the South African mother is described in terms of “balancing maternal and infant benefits” [5]. Certainly we cannot describe the dilemma as a balancing of benefits since the guidelines reflect an attempt to minimize horrible harms to both the mother and infant. Describing the ethical task as one of ‘balancing’ suggests acquiescence to an inherently unjust situation. Characterizing something as an ethical dilemma can have the unintended effect of failing to address a deeper disparity. In the case of the feeding dilemma, it is not just that the dilemma has been falsely placed at the feet of mothers, but that it should not be a dilemma in the first place. The moral reasoning implicit in the divergence between the CDC and WHO guidelines back in 1987 reveals a great deal about what we have been willing to accept in the way of significant social and economic disparities, but even more so, what significant moral disparities we have been willing to tolerate. The barriers standing in the way of more equitable treatment guidelines are extremely significant practical barriers, not natural facts; with great effort and ingenuity, they are nonetheless surmountable barriers. Within dire socio-economic conditions, too often these barriers are seen as overwhelming, and when taken together they can seem so. However, by considering small, targeted, creative solutions coupled with a concerted ramping up of treatment and prevention programs, we can begin to overcome the barriers as we work toward a more ideal solution that is in line with clinical standards in developed countries, standards that represent what is possible.
As we have argued, the best ethical approach to the infant feeding dilemma is not merely to act to minimize the harm done to mother and child in the resource-poor setting, but to work toward a better overall solution that would obviate the need for making such tragic choices. The underlying disparities and the harm to women and children justify: (1) that higher priority be given to solving the infant feeding dilemma in funding decisions and research programs, and (2) support of innovative, community-driven solutions that address the particular economic and cultural challenges that continue to result in HIV-transmission to children within these communities.
Notes
Replacement feeding consists of substituting breast milk with commercial infant formula or home-modified animal milk.
Here are the 2003 guidelines in summary: (1) When replacement feeding is acceptable, feasible, affordable, sustainable and safe, avoidance of all breastfeeding by HIV-infected mothers is recommended; otherwise exclusive breastfeeding is recommended during the first months of life (6 months). (2) To minimize HIV transmission risk, breastfeeding should be discontinued as soon as feasible, taking into account local circumstances, the woman’s situation and the risk of replacement feeding (including infections other than HIV and malnutrition). (3) When HIV-infected mothers choose not to breastfeed from birth or to stop breastfeeding they should be provided with specific guidelines and support for at least the child’s life to ensure adequate replacement feeding. Programs should strive to improve conditions that will make replacement feeding safer for HIV-infected mothers and families. See [30].
This case was provided by Anna Coutsoudis, MD. Dr. Coutsoudis is the Deputy Director of Biomedical Sciences in the Department of Paediatrics & Child Health at the Nelson Mandela School of Medicine affiliated with the University of Kwazulu-Natal. All references to the identity of the woman or child have been omitted.
Paul Ndebele encountered the “blue bag case” while advising at Medical Research Council of Zimbabwe and was involved in its resolution.
According to WHO guidelines for decision makers, other terms have been proposed but not generally accepted. They claim the term is not meant to stigmatize or place blame on the mother. They also say it does not suggest that the mother knows her status (see [30, p. 5]).
References
Mandelbrot, Laurent, Aline Landreau-Mascaro, Claire Rekacewicz, et al., for the Agence Nationale de Recherches sur le SIDA (ANRS) 075 Study Group. 2001. Lamivudine-zidovudine combination for prevention of maternal-infant transmission of HIV-1. Journal of the American Medical Association 285: 2083–2093.
Lallemant, Marc, Gonzague Jourdain, Sophie Le Coeur, et al., for the Perinatal HIV Prevention Trial (Thailand) Investigators. 2004. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. New England Journal of Medicine 351: 217–228.
Dorenbaum, Alejandro, Colleen K. Cunningham, Richard D. Gelber, et al., for the International PACTG 316 Team. 2002. Two-dose intrapartum newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV-1 transmission: A randomized trial. Journal of the American Medical Association 288: 189–198.
World Health Organization. 2006. HIV and infant feeding technical consultation consensus statement. October 25–27, 2006. Geneva: World Health Organization. http://www.who.int/reproductive-health/stis/mtct/infantfeedingconsensusstatement.pdf. Accessed 1 Sept 2007.
Wilfert, Catherine M., and Mary Glenn Fowler. 2007. Balancing maternal and infant benefits and the consequences of breastfeeding in the developing world during the era of HIV infection. Journal of Infectious Disease 195: 165–167.
UNAIDS. 2007. AIDS epidemic update: Special report on HIV/AIDS. http://www.unaids.org/en/KnowledgeCentre/HIVData/EpiUpdate/EpiUpdArchive/2007/default.asp. Accessed 1 Sept 2007.
Inter-Agency Task Team on Mother-to-Child Transmission of HIV. 2000. New data on the prevention of mother-to-child transmission of HIV and their policy implications: Conclusion and recommendations. Technical Consultation UNFPA/UNICEF/WHO/UNAIDS Inter-Agency Task Team on Mother-to-Child Transmission of HIV. Geneva, Switzerland.
Republic of South Africa. Progress report on declaration of commitment on HIV and AIDS.
Wade, Nancy A., Guthrie S. Birkhead, Barbara L. Warren, et al. 1998. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. New England Journal of Medicine 339 (20): 1409–1414.
Cooper, E.R., Man Charurat, Lynne M. Mofenson, and Women and Infants’ Transmission Study Group. 2002. Combination antiretroviral strategies for the treatment of pregnant HIV-1—infected women and prevention of perinatal HIV-1 transmission. Journal of Acquired Immune Deficiency Syndromes 29 (5): 484–494.
Bulterys, M., A. Chao, et al. 1996. Fatal complications after Cesarean section in HIV infected women. AIDS 10 (8): 923–924.
Kilewo, Charles, Katarina Karlsson, Augustine Massawe, et al. 2008. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating infants prophylactically with lamivudine in Dar es Salaam, Tanzania: The Mitra study. Journal of Acquired Immune Deficiency Syndrome 48 (3): 315–323.
Kumwenda, Newton I., Donald R. Hoowever, Lynee M. Mofenson, et al. 2008. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. New England Journal of Medicine 359 (2): 119–129.
Stephenson, Joan. 2008. HIV prevention studies yield mixed results. Journal of the American Medical Association 299 (13): 1529–1530.
Mofenson, Lynee M. 2008. Antiretroviral prophylaxis to reduce breast milk transmission of HIV Type 1: New data but still questions. Journal of Acquired Immune Deficiency Syndrome 48 (3): 237–240.
Dionisio, D., Y. Cao, L. Hongzhou, et al. 2006. Affordable antiretroviral drugs for the under-served markets: How to expand equitable access against the backdrop of challenging scenarios? Current HIV Research 4 (1): 3–20.
Ford, N., D. Wilson, G. Costa Chaves, et al. 2007. Sustaining access to antiretroviral therapy in the less-developed world: Lessons from Brazil and Thailand. AIDS 21 (Supplement 4): S21–S29.
Hymes, Kenneth B., et al. 1981. Kaposi’s sarcoma in homosexual men: A report of eight cases. Lancet 2: 598–600.
Thiry, Lise, Suzanne Sprecher-Goldberger, T. Jonckheer, et al. 1985. Isolation of AIDS virus from cell-free breast milk of three healthy virus carriers. Lancet 2 (8460): 891–892.
Coutsoudis, Anna. 2005. Breastfeeding and the HIV positive mother: The debate continues. Early Human Development 81 (1): 87–93.
Thior, Ibou, Shahin Lockman, and Mashi Study Team. 2006. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs. formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: A randomized trial: The Mashi Study. Journal of the American Medical Association 296 (7): 794–805.
Coutsoudis, Anna, Francois Dabis, and the Breastfeeding and HIV International Transmission Study Group. 2004. Late postnatal transmission of HIV-1 in breast-fed children: An individual patient data meta-analysis. Journal of Infectious Disease 189 (12): 2154–2166.
Leroy, Valeriane, Charlotte Sakarovitch, and the ANRS 1201/1202 Ditrame Plus Study Group. 2007. Acceptability of formula-feeding to prevent HIV postnatal transmission, Abidjan, Cote d’ Ivoire. Epidemiology and Social Science 44 (1): 77–86.
Del Fante, Peter, Francoise Jenniskens, and Louisiana Lush. 1993. HIV, breastfeeding and under-5 mortality: Modeling the impact of policy decisions for and against breastfeeding. Journal of Tropical Medicine and Hygiene 96: 203–211.
Jones, Gareth, Richard W. Steketee, and Bellagio Child Survival Study Group. 2003. How many child deaths can we prevent this year? Lancet 362 (9377): 65–71.
Piwoz, Ellen G., and Jay S. Ross. 2005. Use of population-specific infant mortality rates to inform policy decisions regarding HIV and infant feeding. Journal of Nutrition 135 (5): 1113–1119.
Coutsoudis, Anna. 2005. Breastfeeding and HIV. Best Practice Research in Clinical Obstetrics and Gynaecology 19 (2): 185–196.
Coutsoudis, Anna. 2005. Infant feeding dilemmas created by HIV: South African experiences. Journal of Nutrition 135 (4): 956–959.
Coutsoudis, Anna, Kubendran Pillay, et al. 2005. Routinely available cotrimoxazole prophylaxis and occurrence of respiratory and diarrhoeal morbidity in infants born to HIV-infected mothers in South Africa. South African Medical Journal 95 (5): 339–345.
WHO. 2003. HIV and infant feeding: guidelines for decision-makers. http://www.who.int/nutrition/publications/HIV_IF_decision_maker.pdf. Accessed 1 Sept 2007.
Loder, Asjylyn. 2003. AIDS complicates breastfeeding advice in Africa, in women’s news. Women’s News, New York. http://womensenews.org/article.cfm/dyn/aid/1486/. Accessed 1 Sept 2007.
Farmer, Paul, and Nicole G. Campos. 2004. Rethinking medical ethics: A view from below. Developing World Bioethics 4 (1): 17–41.
Wolf, Leslie E., Bernard Lo, Karen Beckerman, et al. 2001. When parents reject interventions to reduce postnatal human immunodeficiency virus transmission. Archives of Pediatric Adolescent Medicine 155 (8): 927–933.
Gwartney D. Doctors support state in HIV case. Oregonian. April 12, 1999; sec B1, 11.
Seidel, Gill, Vishantie Sewpaul, and Barbara Dano. 2000. Experiences of breastfeeding and vulnerability among a group of HIV-positive women in Durban, South Africa. Health Policy and Planning 15 (1): 24–33.
Shah, Sonal, Nigel C. Rollins, and Child Health Group. 2005. Breastfeeding knowledge among health workers in rural South Africa. Journal of Tropical Pediatrics 51 (1): 33–38.
White, Edith. 1999. Breastfeeding and HIV/AIDS: The research, the politics, the women’s responses. Jefferson: McFarland & Company Inc.
Doherty, Tanya, Mickey Chopra, Lungiswa Nkonki, Debra Jackson, et al. 2006. Effect of the HIV epidemic on infant feeding in South Africa: When they see me coming with the tins they laugh at me. Bulletin of the World Health Organization: 90–96. http://www.who.int/bulletin/volumes/84/2/doherty0206abstract/en/index.html. Accessed 1 Sept 2007.
Chesney, Margaret A., and Ashley W. Smith. 1999. Critical delays in HIV testing and care: The potential role of stigma. American Behavioral Scientist 427: 1162–1174.
Herek, Gregory M., John P. Capitanio, and Keith F. Widaman. 2002. HIV-related stigma and knowledge in the United States: Prevalence and trends, 1991–1999. American Journal of Public Health 92 (3): 371–377.
Fox, Ashley M., Sharon S. Jackson, Nathan B. Hansen, et al. 2007. In their own voices: A qualitative study of women’s risk for intimate partner violence and HIV in South Africa. Violence Against Women 13 (6): 583–602.
Obermeyer, Carla M., and Michelle Osborn. 2007. The utilization of testing and counseling for HIV: A review of the social and behavioral evidence. American Journal of Public Health 97 (10): 1762–1774.
Sastry, Jayagowri, and the SWEN Study Team. 2008. Extended dose nevirapine to 6 weeks of age for infants in Ethiopia, India and Uganda: A randomized trial for prevention of HIV transmission through breastfeeding. In 15th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 3–6 February 2008. Abstract 43.
Ogden, Jessica, and Laura Nyblade. 2005. Common at its core: HIV-related stigma across contexts. International Center for Research on Women (ICRW): Washington, DC. http://www.icrw.org/docs/2005_report_stigma_synthesis.pdf. Accessed 1 Sept 2007.
Castro, Arachu, and Paul Farmer. 2003. Understanding and addressing AIDS-related stigma: From anthropological theory to clinical practice in Haiti. American Journal of Public Health 95: 53–59.
Nattrass, Nicoli. 2004. The moral economy of AIDS in South Africa. Cambridge, UK: Cambridge University Press.
Valdiserri, Ronald O. 2002. HIV/AIDS stigma: An impediment to public health. American Journal of Public Health 92 (3): 341–342.
Ndaba Thoka C., and Catherine Burns. The history and politics of breastfeeding in South Africa in the context of the current HIV driven breastfeeding crisis: Work in progress. South African Medical Research Council, University of Kwazulu-Natal.
Kelley, Maureen, Roshan Patel, and Alice Hazemba. 2008. Addressing barriers to HIV/AIDS prevention among at-risk children and adolescents in Lusaka, Zambia. Study Report for the University of Zambia Department of Community Medicine and the Zambian Ministry of Education. Available upon request: mckelley@u.washington.edu.
UNICEF, South Africa, “UNICEF and PMTCT in South Africa”. http://www.unicef.org/southafrica/hiv_aids_809.html. Accessed 6 Aug 2008.
Bezner, Kerr R., Laifolo Dakishoni, et al. 2008. We grandmothers know plenty: Breastfeeding, complementary feeding and the multifaceted role in grandmothers in Malawi. Social Science and Medicine 66 (5): 1095–1105.
Corneli, Amy L., Ellen G. Piwoz, Margaret E. Bentley, and UNC BAN Study Team. 2007. Involving communities in the design of clinical trial protocols: The BAN Study in Lilongwe, Malawi. Contemporary Clinical Trials 28 (1): 59–67.
Airhihenbuwa, Collins O. 1992. Health promotion and disease prevention strategies for African Americans: A conceptual model. In In health issues in the black community, ed. Ronald L. Braithwaithe, and Sandra E. Taylor, 267–280. San Francisco: Jossey-Bass.
Airhihenbuwa, Collins O., and J.DeWitt Webster. 2004. Culture and African context of HIV/AIDS. Journal of Social Aspects of HIV/AIDS Research Alliance 1 (1): 4–10.
Coovadia, Hoosen, and Anna Coutsoudis. 2007. HIV, infant feeding and survival: Old wine in new bottles but brimming with promise. AIDS 21: 1837–1840.
Kevin M. De Cock, Mary Glenn Fowler, Eric Mercier, et al. 2000. Prevention of mother-to-child HIV transmission in resource-poor countries—translating research into policy and practice. Journal of the American Medical Association 283: 1175–1182.
World Health Organization/UNICEF Consensus Statement. 1992. In the WHO/UNICEF consultation on HIV transmission and breastfeeding. Geneva: WHO.
United Joint Programme on HIV/AIDS. 1996. HIV and infant feeding: An interim statement. Weekly Epidemiological Record 71: 289–291.
Latham, Michael C., and Elizabeth A. Preble. 2000. Appropriate feeding methods for infants of HIV infected mothers in Sub-Saharan Africa. BMJ 320 (7250): 1656–1660.
A question and answer guide: Infant and young child feeding in the context of HIV/AIDS. 2008. http://www.qaproject.org/strat/stratHIVjobaidsintro.htm. Accessed 20 Aug 2007.
Acknowledgements
Support for Faith Fletcher was provided by the Michigan State University (MSU) Program in Bioethics, Humanities, and Society, administered by the Center for Ethics and Humanities in the Life Sciences and the MSU Study Abroad Program in Education, Society and Learning. Paul Ndebele was funded by the National Institutes of Health (NIH) Fogarty international bioethics training program, administered jointly by MSU and University of Malawi’s College of Medicine (R25-TW 7087). The authors wish to thank several audiences and colleagues for helpful feedback and criticism, including: the University of South Carolina Health Promotion, Education and Behavior seminar led by Dr. Edward A. Frongillo, Jr., the HHWG bioethics faculty writing group at the University of Washington, and Dr. Benjamin Wilfond in the Department of Pediatrics, Treuman Katz Center for Bioethics at the University of Washington. We extend special thanks to Dr. Anna Coutsoudis at Nelson R Mandela School of Medicine, University of Kwazulu-Natal, Department of Paediatrics & Child Health for contributing the South African case study and providing insight on the infant feeding dilemma, and to Dr. Susan M. Miller of Weill College of Medicine at Cornell University and Methodist Hospital for helpful feedback on the clinical data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fletcher, F.E., Ndebele, P. & Kelley, M.C. Infant feeding and HIV in Sub-Saharan Africa: what lies beneath the dilemma?. Theor Med Bioeth 29, 307–330 (2008). https://doi.org/10.1007/s11017-008-9083-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11017-008-9083-z