Abstract
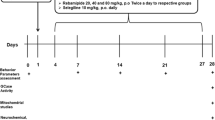
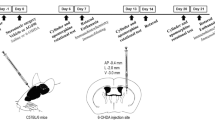
Loss of dopaminergic neurons following Parkinson’s disease (PD) diminishes quality of life in patients. The present study was carried out to investigate the protective effects of simultaneous inhibition of dipeptidyl peptidase-4 (DPP-4) and P2X7 purinoceptors in a PD model and explore possible mechanisms. The 6-hydroxydopamine (6-OHDA) was used as a tool to establish PD model in male Wister rats. The expressions of SIRT1, SIRT3, mTOR, PGC-1α, PTEN, P53 and DNA fragmentation were evaluated by ELISA assay. Behavioral impairments were determined using apomorphine-induced rotational and narrow beam tests. Dopamine synthesis and TH-positive neurons were detected by tyrosine hydroxylase (TH) immunohistochemistry. Neuronal density was determined by Nissl staining. OHDA-lesioned rats exhibited behavioral impairments that reversed by BBG, lin and lin + BBG. We found significant reduced levels of SIRT1, SIRT3, PGC-1α and mTOR in both mid brain and striatum from OHDA-lesioned rats that reversed by BBG, lin and lin + BBG. Likewise, significant increased levels of PTEN and P53 were found in both mid brain and striatum from OHDA-lesioned rats that was reversed by BBG, lin and lin + BBG. TH-positive neurons and neuronal density were markedly reduced OHDA-lesioned rats that reversed by BBG, lin and lin + BBG. Collectively, our results showed protective effects of simultaneous inhibition of DPP-4 and P2X7 purinoceptors in a rat model of PD can be linked to targeting SIRT1/SIRT3, PTEN-mTOR pathways. Moreover, our findings demonstrated that simultaneous inhibition of DPP-4 and P2X7 purinoceptors might have stronger effect on mitochondrial biogenesis compared to only one.





Similar content being viewed by others
Abbreviations
- PD:
-
Parkinson's disease
- DPP-4:
-
dipeptidyl peptidase-4
- 6-OHDA:
-
6-hydroxydopamine
- SIRT1:
-
NAD dependent deacetylase sirtuin-1
- SIRT3:
-
NAD-dependent deacetylase sirtuin-3
- ELISA:
-
enzyme-linked immunosorbent assay
- mTOR:
-
The mammalian target of rapamycin
- PGC-1α:
-
Peroxisome proliferator-activated receptor-γ coactivator1 α
- PTEN:
-
Phosphatase and tensin homolog
- TH:
-
tyrosine hydroxylase
- BBG:
-
brilliant blue G
- lin:
-
linagliptin
- SNC:
-
nigra pars compacta
- NF-κB:
-
nuclear factor kappa B
- Nrf2:
-
Nuclear factor erythroid-2-related factor 2
- FOXO3a:
-
Forkhead box class O 3a
References
Abdelsalam RM, Safar MM (2015) Neuroprotective effects of vildagliptin in rat rotenone Parkinson's disease model: role of RAGE-NF κB and Nrf2-antioxidant signaling pathways. J Neurochem 133:700–707
Ajami M et al (2017) Therapeutic role of sirtuins in neurodegenerative disease and their modulation by polyphenols. Neuroscience & Biobehavioral Reviews 73:39–47
Amani H, Arzaghi H, Bayandori M, Dezfuli AS, Pazoki-Toroudi H, Shafiee A, Moradi L (2019a) Controlling cell behavior through the Design of Biomaterial Surfaces: a focus on surface modification techniques advanced materials interfaces:1900572
Amani H, Habibey R, Shokri F, Hajmiresmail SJ, Akhavan O, Mashaghi A, Pazoki-Toroudi H (2019b) Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Scientific reports 9:6044
Amani H, Kazerooni H, Hassanpoor H, Akbarzadeh A, Pazoki-Toroudi H (2019c) Tailoring synthetic polymeric biomaterials towards nerve tissue engineering: a review. Artificial cells, nanomedicine, and biotechnology 47:3524–3539
Amani H, Mostafavi E, Alebouyeh MR, Arzaghi H, Akbarzadeh A, Pazoki-Toroudi H, Webster TJ (2019d) Would colloidal gold nanocarriers present an effective diagnosis or treatment for ischemic stroke? Int J Nanomedicine 14:8013
Amani H et al (2018) Three-dimensional graphene foams: synthesis, properties, biocompatibility, biodegradability, and applications in tissue engineering. ACS Biomaterials Science & Engineering 5:193–214
Baluchnejadmojarad T, Jamali-Raeufy N, Zabihnejad S, Rabiee N, Roghani M (2017) Troxerutin exerts neuroprotection in 6-hydroxydopamine lesion rat model of Parkinson’s disease: possible involvement of PI3K/ERβ signaling. Eur J Pharmacol 801:72–78
Baluchnejadmojarad T, Roghani M, Nadoushan MRJ, Bagheri M (2009) Neuroprotective effect of genistein in 6-hydroxydopamine hemi-parkinsonian rat model Phytotherapy research: an international journal devoted to pharmacological and toxicological evaluation of natural product derivatives 23:132-135
Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell and tissue research 318:121–134
Cruces-Sande A, Rodríguez-Pérez AI, Herbello-Hermelo P, Bermejo-Barrera P, Méndez-Álvarez E, Labandeira-García JL, Soto-Otero R (2019) Copper increases brain oxidative stress and enhances the ability of 6-Hydroxydopamine to cause dopaminergic degeneration in a rat model of Parkinson’s disease. Mol Neurobiol 56:2845–2854
Deumens R, Blokland A, Prickaerts J (2002) Modeling Parkinson's disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol 175:303–317
Ge H, Yan Z, Zhu H, Zhao H (2019) MiR-410 exerts neuroprotective effects in a cellular model of Parkinson's disease induced by 6-hydroxydopamine via inhibiting the PTEN/AKT/mTOR signaling pathway. Experimental and molecular pathology 109:16–24
Geng L, Zhang T, Liu W, Chen Y (2018) miR-494-3p modulates the progression of in vitro and in vivo Parkinson’s disease models by targeting SIRT3. Neuroscience letters 675:23–30
Ghosh HS, McBurney M, Robbins PD (2010) SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One 5:e9199
Gomez-Lazaro M, Galindo MF, Concannon CG, Segura MF, Fernandez-Gomez FJ, Llecha N, Comella JX, Prehn JH, Jordan J (2008) 6-Hydroxydopamine activates the mitochondrial apoptosis pathway through p38 MAPK-mediated, p53-independent activation of Bax and PUMA. J Neurochem 104:1599–1612
Hall S, Janelidze S, Surova Y, Widner H, Zetterberg H, Hansson O (2018) Cerebrospinal fluid concentrations of inflammatory markers in Parkinson’s disease and atypical parkinsonian disorders. Scientific reports 8:13276
Hasty P, Sharp ZD, Curiel TJ, Campisi J (2013) mTORC1 and p53: clash of the gods? Cell Cycle 12:20–25
Jiang M et al (2012) Neuroprotective role of Sirt1 in mammalian models of Huntington's disease through activation of multiple Sirt1 targets. Nat Med 18:153
Kiasalari Z, Baluchnejadmojarad T, Roghani M (2016) Hypericum perforatum hydroalcoholic extract mitigates motor dysfunction and is neuroprotective in intrastriatal 6-Hydroxydopamine rat model of Parkinson’s disease. Cell Mol Neurobiol 36:521–530
Kim D et al (2007) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J 26:3169–3179
Lang AE, Lozano AM (1998) Parkinson's disease New England Journal of Medicine 339:1130–1143
Li W, Jiang Y, Wang Y, Yang S, Bi X, Pan X, Ma A (2018) MiR-181b regulates autophagy in a model of Parkinson’s disease by targeting the PTEN/Akt/mTOR signaling pathway. Neurosci Lett 675:83–88
Liu L, Peritore C, Ginsberg J, Kayhan M, Donmez G (2015) SIRT3 attenuates MPTP-induced nigrostriatal degeneration via enhancing mitochondrial antioxidant capacity. Neurochemical research 40:600–608
Martins I (2017a) Nutrition therapy regulates caffeine metabolism with relevance to NAFLD and induction of type 3 diabetes. J diabetes Metab Disord 4:019
Martins IJ (2017b) Single gene inactivation with implications to diabetes and multiple organ dysfunction syndrome. J Clin Epigenet 3:24
Obeso JA, Olanow CW, Nutt JG (2000) Levodopa motor complications in Parkinson's disease. Elsevier,
Paxinos G, Franklin KB (2019) Paxinos and Franklin's the mouse brain in stereotaxic coordinates. Academic press,
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic New York
Pillai VB, Sundaresan NR, Gupta MP (2014) Regulation of Akt signaling by sirtuins: its implication in cardiac hypertrophy and aging. Circulation research 114:368–378
Roghani M, Behzadi G, Baluchnejadmojarad T (2002) Efficacy of elevated body swing test in the early model of Parkinson's disease in rat. Physiology & behavior 76:507–510
Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell 168:960–976
Schwarting R, Huston J (1997) Behavioral and neurochemical dynamics of neurotoxic meso-striatal dopamine lesions. Neurotoxicology 18:689–708
Singh P, Hanson PS, Morris CM (2017) SIRT1 ameliorates oxidative stress induced neural cell death and is down-regulated in Parkinson’s disease. BMC neuroscience 18:–46
Stambolic V et al (2001) Regulation of PTEN transcription by p53. Molecular cell 8:317–325
Sveinbjornsdottir S (2016) The clinical symptoms of Parkinson's disease. J Neurochem 139:318–324
Wang CY, Sun ZN, Wang MX, Zhang C (2018) SIRT1 mediates salidroside-elicited protective effects against MPP+-induced apoptosis and oxidative stress in SH-SY5Y cells: involvement in suppressing MAPK pathways. Cell biology international 42:84–94
Wang Q, Li L, Li C, Pei Z, Zhou M, Li N (2015) SIRT3 protects cells from hypoxia via PGC-1α-and MnSOD-dependent pathways. Neuroscience 286:109–121
Wang XH, Xie X, Luo XG, Shang H, He ZY (2017) Inhibiting purinergic P2X7 receptors with the antagonist brilliant blue G is neuroprotective in an intranigral lipopolysaccharide animal model of Parkinson's disease. Mol Med Rep 15:768–776
Wang Z, Sun L, Jia K, Wang H, Wang X (2019) miR-9-5p modulates the progression of Parkinson’s disease by targeting SIRT1. Neuroscience letters 701:226–233
Zhang F, Wang S, Gan L, Vosler PS, Gao Y, Zigmond MJ, Chen J (2011) Protective effects and mechanisms of sirtuins in the nervous system. Progress in neurobiology 95:373–395
Zhang J-Y, Deng Y-N, Zhang M, Su H, Qu Q-M (2016) SIRT3 acts as a neuroprotective agent in rotenone-induced Parkinson cell model. Neurochemical research 41:1761–1773
Acknowledgments
The present study was financially supported by research affairs of Iran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jamali-Raeufy, N., Mojarrab, Z., Baluchnejadmojarad, T. et al. The effects simultaneous inhibition of dipeptidyl peptidase-4 and P2X7 purinoceptors in an in vivo Parkinson’s disease model. Metab Brain Dis 35, 539–548 (2020). https://doi.org/10.1007/s11011-020-00538-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-020-00538-x