Abstract
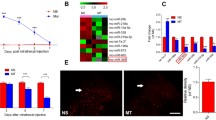
Chronic exposure to morphine can produce tolerance, dependence and addiction, but the underlying neurobiological basis is still incompletely understood. c-Jun, as an important component of the activator protein-1 transcription factor, is supposed to take part in regulating gene expression in AC/cAMP/PKA signaling. MicroRNA (miRNA) has emerged as a critical regulator of neuronal functions. Although a number of miRNAs have been reported to regulate the μ-opioid receptor expression, there has been no report about miRNAs to regulate chronic morphine-induced, naloxone-precipitated cAMP overshoot. Our results showed that chronic morphine pretreatment induced naloxone-precipitated cAMP overshoot in concentration- and time-dependent manners in HEK 293/μ cells. Chronic morphine pretreatment alone elevated both c-Jun protein and miR-139-5p expression levels, while dramatically artificial elevation of miR-139-5p inhibited c-Jun at the translational level. Furthermore, dramatically artificial upregulation of intracellular miR-139-5p limited chronic morphine-induced, naloxone-precipitated cAMP overshoot. These findings suggested that miR-139-5p was involved in regulating chronic morphine-induced, naloxone-precipitated cAMP overshoot in a negative feedback manner through its target c-Jun, which extends our understanding of neurobiological mechanisms underlying morphine dependence and addiction.



Similar content being viewed by others
References
Bonci A, Williams JT (1997) Increased probability of GABA release during withdrawal from morphine. J Neurosci 17:796–803
Chan P, Lutfy K (2016) Molecular Changes in Opioid Addiction: The Role of Adenylyl Cyclase and cAMP/PKA System. Prog Mol Biol Transl Sci 137:203–227
Couceyro P, Douglass J (1995) Precipitated morphine withdrawal stimulates multiple activator protein-1 signaling pathways in rat brain. Mol Pharmacol 47:29–39
Cunha FQ, Teixeira MM, Ferreira SH (1999) Pharmacological modulation of secondary mediator systems--cyclic AMP and cyclic GMP--on inflammatory hyperalgesia. Br J Pharmacol 127:671–678
Dave RS, Khalili K (2010) Morphine treatment of human monocyte-derived macrophages induces differential miRNA and protein expression: impact on inflammation and oxidative stress in the central nervous system. J Cell Biochem 110:834–845
Duman RS, Tallman JF, Nestler EJ (1988) Acute and chronic opiate-regulation of adenylate cyclase in brain: specific effects in locus coeruleus. J Pharmacol Exp Ther 246:1033–1039
El Kouhen R, Kouhen OM, Law PY, Loh HH (1999) The absence of a direct correlation between the loss of [D-Ala2, MePhe4,Gly5-ol]Enkephalin inhibition of adenylyl cyclase activity and agonist-induced mu-opioid receptor phosphorylation. J Biol Chem 274:9207–9215
Fan P, Jiang Z, Diamond I, Yao L (2009) Up-regulation of AGS3 during morphine withdrawal promotes cAMP superactivation via adenylyl cyclase 5 and 7 in rat nucleus accumbens/striatal neurons. Mol Pharmacol 76:526–533
Gach K, Piestrzeniewicz M, Fichna J, Stefanska B, Szemraj J, Janecka A (2008) Opioid-induced regulation of mu-opioid receptor gene expression in the MCF-7 breast cancer cell line. Biochem Cell Biol 86:217–226
Glatt CE, Snyder SH (1993) Cloning and expression of an adenylyl cyclase localized to the corpus striatum. Nature 361:536–538
Gonzalez-Nunez V, Noriega-Prieto JA, Rodriguez RE (2014) Morphine modulates cell proliferation through mir133b &mir128 in the neuroblastoma SH-SY5Y cell line. Biochim Biophys Acta 1842:566–572
Guitart X, Thompson MA, Mirante CK, Greenberg ME, Nestler EJ (1992) Regulation of cyclic AMP response element-binding protein (CREB) phosphorylation by acute and chronic morphine in the rat locus coeruleus. J Neurochem 58:1168–1171
He Y, Yang C, Kirkmire CM, Wang ZJ (2010) Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J Neurosci 30:10251–10258
Hwang CK, Wagley Y, Law PY, Wei LN, Loh HH (2012) MicroRNAs in opioid pharmacology. J NeuroImmune Pharmacol 7:808–819
Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598
Kim KS, Lee KW, Lee KW, Im JY, Yoo JY, Kim SW, Lee JK, Nestler EJ, Han PL (2006) Adenylyl cyclase type 5 (AC5) is an essential mediator of morphine action. Proc Natl Acad Sci U S A 103:3908–3913
Kim JA, Bartlett S, He L, Nielsen CK, Chang AM, Kharazia V, Waldhoer M, Ou CJ, Taylor S, Ferwerda M, Cado D, Whistler JL (2008) Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence. Curr Biol 18:129–135
Lane-Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK, Nestler EJ (1997) CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J Neurosci 17:7890–7901
Law PY, Wong YH, Loh HH (2000) Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol 40:389–430
Li F, Wu N, Su RB, Liu Y, Lu XQ, Li J (2009) Comparison of agmatine with moxonidine and rilmenidine in morphine dependence in vitro: role of imidazoline I(1) receptors. Eur J Pharmacol 612:1–8
Li F, Ma H, Wu N, Li J (2016) IRAS Modulates Opioid Tolerance and Dependence by Regulating mu Opioid Receptor Trafficking. Mol Neurobiol 53:4918–4930
Lu Z, Xu J, Xu M, Pasternak GW, Pan YX (2014) Morphine regulates expression of mu-opioid receptor MOR-1A, an intron-retention carboxyl terminal splice variant of the mu-opioid receptor (OPRM1) gene via miR-103/miR-107. Mol Pharmacol 85:368–380
Madhavan A, He L, Stuber GD, Bonci A, Whistler JL (2010) micro-Opioid receptor endocytosis prevents adaptations in ventral tegmental area GABA transmission induced during naloxone-precipitated morphine withdrawal. J Neurosci 30:3276–3286
Manna PR, Stocco DM (2007) Crosstalk of CREB and Fos/Jun on a single cis-element: transcriptional repression of the steroidogenic acute regulatory protein gene. J Mol Endocrinol 39:261–277
Nestler EJ (2004) Historical review: Molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci 25:210–218
Nestler EJ, Aghajanian GK (1997) Molecular and cellular basis of addiction. Science 278:58–63
Sanchez-Simon FM, Zhang XX, Loh HH, Law PY, Rodriguez RE (2010) Morphine regulates dopaminergic neuron differentiation via miR-133b. Mol Pharmacol 78:935–942
Sassone-Corsi P, Ransone LJ, Verma IM (1990) Cross-talk in signal transduction: TPA-inducible factor jun/AP-1 activates cAMP-responsive enhancer elements. Oncogene 5:427–431
Sharma SK, Klee WA, Nirenberg M (1975) Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci U S A 72:3092–3096
Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ (1991) A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res 548:100–110
Widnell KL, Russell DS, Nestler EJ (1994) Regulation of expression of cAMP response element-binding protein in the locus coeruleus in vivo and in a locus coeruleus-like cell line in vitro. Proc Natl Acad Sci U S A 91:10947–10951
Wu Q, Law PY, Wei LN, Loh HH (2008) Post-transcriptional regulation of mouse mu opioid receptor (MOR1) via its 3’ untranslated region: a role for microRNA23b. FASEB J 22:4085–4095
Wu Q, Zhang L, Law PY, Wei LN, Loh HH (2009) Long-term morphine treatment decreases the association of mu-opioid receptor (MOR1) mRNA with polysomes through miRNA23b. Mol Pharmacol 75:744–750
Wu Q, Hwang CK, Zheng H, Wagley Y, Lin HY, Kim DK, Law PY, Loh HH, Wei LN (2013) MicroRNA 339 down-regulates mu-opioid receptor at the post-transcriptional level in response to opioid treatment. FASEB J 27:522–535
Zhang Y, Shen WL, Shi ML, Zhang LZ, Zhang Z, Li P, Xing LY, Luo FY, Sun Q, Zheng XF, Yang X, Zhao ZH (2015) Involvement of aberrant miR-139/Jun feedback loop in human gastric cancer. Biochim Biophys Acta 1853:481–488
Zheng H, Zeng Y, Zhang X, Chu J, Loh HH, Law PY (2010) mu-Opioid receptor agonists differentially regulate the expression of miR-190 and NeuroD. Mol Pharmacol 77:102–109
Acknowledgements
This work was supported by the National Basic Research Program of China (2015CB553504), National Key R&D Program of China (2017YFC1310404) and the National Natural Science Foundation of China (Nos. 81571302 and U1502225).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cao, DN., Shi, JJ., Wu, N. et al. Modulation of miR-139-5p on chronic morphine-induced, naloxone-precipitated cAMP overshoot in vitro. Metab Brain Dis 33, 1501–1508 (2018). https://doi.org/10.1007/s11011-018-0257-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0257-8